* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 6. Absorption of Heat
Chemical thermodynamics wikipedia , lookup
Insulated glazing wikipedia , lookup
Thermal conductivity wikipedia , lookup
Dynamic insulation wikipedia , lookup
Temperature wikipedia , lookup
Calorimetry wikipedia , lookup
Heat capacity wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat equation wikipedia , lookup
Thermoregulation wikipedia , lookup
Thermodynamic system wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Thermal radiation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Countercurrent exchange wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Heat transfer wikipedia , lookup
Adiabatic process wikipedia , lookup
Thermal conduction wikipedia , lookup
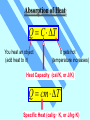
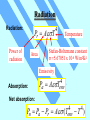
Unit 3 Temperature, Heat, and the First Law of Thermodynamics Absorption of Heat Q C T You heat an object (add heat to it) It gets hot (temperature increases) Heat Capacity (cal/K, or J/K) Q cm T Specific Heat (cal/g · K, or J/kg·K) By definition of heat, the specific heat of water is c = 1 cal/g · K higher than most other substance Latent Heat During some phase transitions (i.e. ice - water), heating does not lead to increase of temperature until the transition is completed The thermal energy required for the transition: Q mL Latent Heat (cal/g, or J/kg) Work Done During Volume Change Consider a gas cylinder of piston area A, gas pressure p, and gas volume V The gas expands, the piston moves by ds, and the volume changes from V to V+dV=V+A·ds The work done BY the gas: dW=F·ds=A·p·ds=p·dV The work done BY the gas during the volume change from Vi to Vf Vf W p dV Vi P-V Diagram Vf W p dV Vi is the area under the curve in the p-V diagram representing a path from Vi to Vf (Pay attention to the direction of the path!!) Close cycle W = enclosed area Same Vi and Vf, different path different area different work The First Law of Thermodynamics For given initial and final points, Q - W is the same for all paths. The First Law of Thermodynamics: Eint Q W difference of internal energy heat added to the system work done by the system The First Law of Thermodynamics Eint Q W or dEint dQ dW or dEint dQ dWon Infinitesimal process work done ON the system The change of internal energy is path independent The internal energy is a state function Eint Q W Adiabatic process: Q=0 Cyclic process: ∆Eint = 0 ∆Eint = -W Q=W = Area enclosed by the cycle Free expansion: Q = 0, W = 0 Isovolumetric process: W=0 ∆Eint = 0 ∆Eint = Q Heat Transfer Mechanisms Conduction – through the materials Convection – through the movement of a heated substance Radiation – through emission of electromagnetic field Conduction Exchange of kinetic energy – Between molecules or atoms (insulators) – By “free electrons” (metals) Q dT H kA t dx Rate of heat transfer Thermal conductivity (W/m K) Temperature gradient Cross-section Convection Heat transfer by the movement of a heated substance. Natural convection Result from difference in density Forced convection The heated substance is forced to move Radiation Radiation: Pr AT Power of radiation 4 Temperature Stefan-Boltzmann constant 5.67051 x 10-8 W/m2K4 Area Emissivity Absorption: Pa 4 ATenv Net absorption: Pn Pa Pr 4 A (Tenv 4 T ) HRW 54E (5th ed.). A 150 g copper bowl contains 220 g of water, both at 20.0˚C. A very hot 300 g copper cylinder is dropped into the water, causing the water to boil, with 5.00 g being converted to steam. The final temperature of the system is 100˚C. (a) How much heat was transferred to the water? (b) How much to the bowl? (c) What was the original temperature of the cylinder? (a) The heat transferred to the water of mass ml is: Qw = cwmw∆T + LVms = (1 cal/gC˚)(220g)(100˚C-20.0˚C)+(539 cal/g)(5.00 g) = 20.3 kcal Q mL Q cm T HRW 54E (5th ed.). A 150 g copper bowl contains 220 g of water, both at 20.0˚C. A very hot 300 g copper cylinder is dropped into the water, causing the water to boil, with 5.00 g being converted to steam. The final temperature of the system is 100˚C. (a) How much heat was transferred to the water? (b) How much to the bowl? (c) What was the original temperature of the cylinder? (b) The heat transferred to the bowl is: Qb = cbmb∆T = (0.0923 cal/gC˚)(150g)(100˚C-20.0˚C)= 1.11 kcal (c) Let it be Ti, then -Qw - Qb = ccmc(Tf-Ti) Ti Qw Qb T f 873˚C cc mc Q mL Q cm T HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the process is a complete cycle (beginning and ending in the same thermodynamic state), ∆Eint = 0 and Q = W, QAB + QBC + QCA = W QCA = W- QAB - QBC = 15.0 J - 20.0 J - 0 = -5.0 J 5.0 J of energy leaves the gas in the form of heat. Eint Q W HRW 84E (5th ed.). A cylindrical copper rod of length 1.2 m and cross-sectional area 4.8 cm2 is insulated to prevent heat loss through its surface. The ends are maintained at a temperature difference of 100˚C by having one end in a water-ice mixture and the other in boiling water and steam. (a) Find the rate at which heat is conducted along the rod. (b) Find the rate at which ice melts at the cold end. (a) The rate at which the heat is conducted along the rod kA(TH TC ) H L 16 J/s 401 W/m K 4.8 10 -4 m2 100 C 1.2 m (b) The rate at which the ice melts is dm H 16 J/s 0.048 g/s dt L 333 J/g Q mL H Q dT kA t dx