* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Experiment 7: Determination of the concentration of a solution of an
Water splitting wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
History of electrochemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Diamond anvil cell wikipedia , lookup
Acid strength wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Stoichiometry wikipedia , lookup
Crystallization wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Electrolysis of water wikipedia , lookup
Acid–base reaction wikipedia , lookup
SCHOOL OF CHEMISTRY UNIVERSITY OF KWAZULU‐NATAL DURBAN CENTRE General Principles of Chemistry CHEM 110 LABORATORY MANUAL 1st SEMESTER 2009 1
UNIVERSITY OF KWAZULU‐NATAL DURBAN CENTRE SCHOOL OF CHEMISTRY I, the undersigned (please print your full name): ___________________________________________________________________ Student Number: ____________________ do hereby acknowledge having read and understood the documents headed Occupational Health and Safety and Laboratory Regulations. Furthermore, I accept that contravention of these rules and regulations may lead to my expulsion from the laboratory class, or classes, with subsequent loss of my Duly Performed (DP) certificate. I agree to abide by any additional laboratory regulations or safety rules presented in writing in the laboratory manuals/books or issued verbally by the lecturer‐in‐charge, or other responsible member of staff, during pre‐laboratory lectures or in the laboratory. In addition, I understand that I must attend at least 80% of the scheduled laboratory classes and that failure to do so, irrespective of the reasons, may result in the loss of my DP certificate. DATE: ___________________________ SIGNATURE: ___________________________ 2
Table of contents Occupational Health And Safety .................................................................................................... 3 Laboratory Regulations .................................................................................................................... 4 General Advice .................................................................................................................................. 4 Safety Precautions ............................................................................................................................. 6 General Fire Orders .......................................................................................................................... 8 Experiment 1: Introductory laboratory techniques ................................................................... 9 Experiment 2: Preparation of copper metal from copper sulfate pentahydrate ................. 17 Experiment 3: An introduction to volumetric analysis ........................................................... 21 Experiment 4: Determination of carbonate in an antacid tablet ............................................ 27 Experiment 5: Preparation of a primary standard solution ................................................... 34 Experiment 6: Determination of acetic acid in vinegar ........................................................... 42 Experiment 7: Determination of the concentration of a solution of an ammonium salt .... 48 Experiment 8: Determination of the percentage purity of a sample of Mohr’s salt ........... 54 Experiment 9: Determination of the molar mass of magnesium ........................................... 60 Experiment 10a: The absolute zero of temperature ................................................................. 67 Experiment 10b: Calorimetry: Determination of the heat of neutralisation ........................ 75 Experiment 11: pH and indicators ........................................................................................... 85 Experiment 12: Chemical Kinetics: Rates of chemical reactions .......................................... 94 Appendix 1: Laboratory apparatus ........................................................................................... 100 Appendix 2: The laboratory balance ......................................................................................... 103 Appendix 3: Volumetric apparatus ........................................................................................... 104 Appendix 4: Experimental errors .............................................................................................. 109 Appendix 5: The elements .......................................................................................................... 117 Health and Safety 3
Occupational Health and Safety You are warned that all substances handled and all operations performed in a laboratory can be hazardous or potentially hazardous. All substances must be handled with care and disposed of according to laid down procedures. All operations and manipulations must be carried out in an organised and attentive manner. In order to assist you in developing good and safe laboratory techniques, a set of Laboratory Rules and Regulations is attached. You are required to read these and to acknowledge that you have read and understood them. Additionally, in the laboratory manuals/books and/or pre‐laboratory lectures your attention will be drawn to the correct and safe handling of specific chemicals/reagents/solvents, and to the correct/safe manner in which specified laboratory operations must be carried out. These specific instructions and/or warnings must never be ignored. It is a legal requirement that SAFETY GLASSES, LABORATORY COATS and CLOSED SHOES are worn in the laboratory at all times. Note: 1. Sunglasses (normal or prescription) may NOT be worn as a substitute for safety glasses. Prescription glasses (except sunglasses) are acceptable, but MUST be worn at ALL TIMES. 2. Some types of contact lenses should not be worn in the laboratory. Students who wear contact lenses must check the risk factor with their lens supplier. 3. In addition to being closed, shoes must be sensible – HIGH HEELED SHOES are HAZARDOUS. 4. The School requires students to remove, or to make safe, headgear that is considered dangerous or a potential hazard. 5. The School requires students with long hair to tie it back. Health and Safety 4
Laboratory Regulations 1. Students shall present themselves ten minutes before the start of each scheduled laboratory session. Latecomers will be refused entry to the laboratory. 2. No student is permitted to work in the laboratory outside scheduled laboratory hours. 3. Students are not allowed to enter the preparation room, which is located along the side of the laboratory. If reagent bottles need to be refilled, broken apparatus replaced, etc., students should request assistance from a demonstrator. 4. Apparatus and chemicals are NOT to be removed from the laboratory. 5. You will find the laboratory bench clean upon your arrival, and it should be clean when you leave the laboratory. Bench tops should be wiped and glassware and other apparatus should be left clean and dry. 6. All solids must be discarded in the bins at the outer ends of each bench. Do not throw matches, paper, or any insoluble chemicals into the sink. Liquids must be discarded into the ceramic sinks or designated disposal bottles. 7. All students are required to wear a laboratory coat, and no student will be permitted to work in the laboratory without one. 8. All students who do not wear conventional spectacles must wear a pair of safety spectacles. No student will be permitted to work in the laboratory without eye protection. 9. No food or drink is allowed in the laboratory. Eating is not permitted in the laboratory. 10. Cell phones must be switched off whilst you are in the laboratory. General Advice In order to work quickly and accurately, students should carefully plan their work before coming into the laboratory. A schedule of the experiments to be performed will be posted Health and Safety 5
on the notice board and students are expected to read the relevant portions of the notes in their laboratory manuals before their practical session. The pre‐laboratory problems on the green sheets should be completed at home prior to the relevant laboratory. These exercises are designed to familiarise you with certain aspects of the theory of the experiment you are to carry out, as well as giving you practice in the calculations involved. It is thus very important that you complete them before coming to the laboratory. Answers to questions and numerical problems are given to allow you to check that you are getting them correct. This is most beneficial if you first attempt to answer the questions yourself, and then only look at the answers to see if you are right, rather than merely consulting the answers directly. Being able to follow and understand when someone else does a calculation does not teach you how to do it yourself! These exercises are not marked, but the laboratory exercises themselves, which are marked, will contain questions which are very similar to those found in the pre‐lab exercises. These exercises thus serve as preparation for the laboratory exercises, and it is thus in your own interest to ensure that you have mastered the material. You will not be allowed entry into the laboratory unless you have completed your green sheet beforehand. You must record all your results neatly in ink on the sheets provided. If you forget your laboratory manual, borrow a friend’s and make a copy before coming to the laboratory. All results sheets for a particular laboratory must be handed in at the end of that session; students who do not do so will be deemed to have been absent, with possible subsequent DP implications. All absences from practicals will automatically be graded as 0 unless a suitable written excuse (medical or other) is furnished. Written excuses should be provided within one week of re‐attendance, or they will not be accepted. Under special circumstances a student may be allowed to attend a laboratory session other than their allotted one, thereby allowing them to “miss” their normal session without being penalised. However, driver’s‐license‐appointments, leaving‐campus‐early‐
for‐vacations, and studying‐for‐tests‐the‐following‐day do not constitute valid reasons. Students who feel their reasons are valid should see the lecturer‐in‐charge beforehand to make arrangements. Please keep in mind that a DP certificate will be refused to any student who has not attended the required minimum number (80%) of laboratory sessions, irrespective of the reasons for absences. Health and Safety 6
Safety Precautions The chemical laboratory is not a place for horseplay. Do not attempt unauthorised experiments or practical jokes on your neighbour. Such activities are dangerous and can cause serious injuries. Report all accidents, cuts, burns, etc. ‐ however minor ‐ to your demonstrator or staff member in charge. Eyewash stations are located in several places in the laboratory. See that you know where the nearest one to your bench is located in case of an accident. Liquids – whether corrosive or not – must be handled with care, and spilling on the bench or floor should be carefully avoided. Any spillage must be cleaned up at once. If a corrosive liquid, such as an acid or base, is spilled, call your demonstrator or the staff member in charge. Reagent bottles must be stoppered immediately after use and returned to their correct place. It is absolutely forbidden to introduce anything into reagent bottles, and solutions taken from reagent bottles should never be returned to the bottles. Do not lay the stopper of a reagent bottle on the desktop – it could become contaminated. The correct procedure for pouring liquids from reagent bottles is described below. Hold the stopper in the bottle, and tilt the bottle slightly to wet the stopper. This lubricates the ground glass and makes removing the stopper easier. Moisten the inside of the neck and the lip with the stopper. This stops the first drops from gushing out when pouring. Replace the stopper. Remove the stopper again by turning your hand over and holding the stopper between two fingers. The neck of the bottle should touch the edge of the vessel you are pouring into to prevent liquid from running down the outside of the bottle. The stopper must remain firmly held between the fingers whilst pouring the liquid. Replace the stopper when enough liquid has been poured out. Health and Safety 7
Do not heat graduated cylinders or bottles because they can easily break. Heat all other apparatus gently at first to avoid breakage. Do not put anything in your mouth while working in the laboratory, nor taste chemicals or solutions! Breakages of expensive items of glassware such as burettes, pipettes, thermometers, graduated cylinders, etc. will be charged for. Examination results can be withheld at the end of a semester until such charges have been settled. Health and Safety 8
General Fire Orders These orders should be read in conjunction with any fire fighting instructions that are displayed in the laboratory. In the event of a fire: Alert your demonstrator or staff member in charge (if they haven’t already noticed…) and obey any instructions that they give you. On hearing a fire evacuation alarm: Stop normal work immediately. Make any apparatus safe – turn off Bunsen burners, stirrers, vacuum pumps, etc. Unless your demonstrator or staff member in charge has given you any other special instructions, follow the green emergency exit signs out of the building. Assemble on the grassed area between J and L blocks. You should make sure that you know the location of the fire extinguisher in your lab. Experiment 1 9
Experiment 1: Introductory laboratory techniques: Determination of densities of solids and liquids AIM The purpose of this laboratory exercise is threefold: to introduce laboratory regulations, equipment and correct procedures, to illustrate some elementary manipulative techniques and the use of the three decimal place balance, and to give practice in the manipulation of significant figures. INTRODUCTION Density, d, is defined as the ratio of the two properties, mass, m, and volume, V, i.e. m
d = in units of g cm‐3 or kg m‐3 (for solids and liquids), or g dm‐3 (for gases). V
The mass of a sample is independent of external conditions such as temperature or pressure, while the volume is sensitive to temperature. This is particularly true for gases. Density is a physical property of a material, and is characteristic of that material. A pure material can be identified by comparison of its measured density with the accepted values found in standard reference tables. When a material is to be identified in this way, it is very important to ensure that the material is as pure as possible, and that the value is measured as accurately as possible. EXPERIMENTAL PROCEDURE Laboratory equipment Check the equipment in your locker against the list provided (Appendix 1). Make sure you know the names of the various pieces of equipment in your locker, and that you know which piece of equipment corresponds to each item on your locker list. Exercise in weighing Once your demonstrator has shown you how to use the three decimal place balance, weigh a dry 50 cm3 beaker. Measure 25 cm3 of water into the beaker using a 10 cm3 measuring cylinder, and weigh the beaker and water. Record both masses. Calculate the mass of water in the beaker by difference. Empty and dry the beaker. Now measure 25 cm3 of water into the beaker using a 50 cm3 measuring cylinder. Calculate the mass of water in the beaker by difference. Experiment 1 10
You will be given the density of water at the temperature of the laboratory. Use the density and the mass of water to determine the volume of water measured in each case. Determination of Density Densities of Liquids You are provided with two liquids A and B. My measuring the densities of these two liquids you will be able to identify which is the regular soft drink and which is the diet soft drink. Weigh a clean, dry 50 cm3 graduated measuring cylinder and record the mass. Measure 25.0 cm3 of liquid A into the cylinder, make sure that the outside of the cylinder is dry, and then re‐weigh the cylinder and its contents. Record the volume and mass, as before, and from these data calculate the density of liquid A. Discard the liquid into the sink, rinse the measuring cylinder with acetone, and dry. Discard the acetone into the waste bottle provided. Repeat the above procedure for liquid B. Record the results, to the correct number of significant figures, on your report sheet. Identify which of A and B is the diet soft drink. Densities of Solids Weigh a clean, dry, 50 cm3 beaker, add ~15 g of solid C and re‐weigh. Record both masses. Place 5.0 cm3 of water in the 10 cm3 graduated measuring cylinder, then tilt the measuring cylinder and carefully slide the solid into the liquid. Dislodge air bubbles by gently tapping the cylinder. Reread the volume to the nearest 0.1 cm3. Determine the volume of the solid from the difference between the two readings, and calculate the density and percentage error. Discard the solid into the waste bucket provided, rinse the measuring cylinder with acetone, and dry. Repeat the procedure with ~26 g of solid D, and report both results to the correct number of significant figures. Calculation of % error The difference between the experimentally determined value and the reference (or literature) value is best expressed as a percentage, which is always quoted as a positive value. ⎡ dlit - dexp ⎤
% error = ⎢
× 100 ⎣ dlit ⎥⎦
The reference values for the solids you will have looked up for yourself as part of the pre‐
laboratory exercise. Experiment 1 11
Cleaning Up Rinse all glassware used and replace in the correct locker. When you are ready to go, call your demonstrator to your bench. If all is satisfactory, he or she will accept and initial your report, and you may then leave. Experiment 1 12
Experiment 1: Introductory laboratory techniques: Determination of densities of solids and liquids PRE‐LABORATORY EXERCISE 1. Look up the densities of lead (Pb) and zinc (Zn) in your textbook and enter on your answer sheet. 2. a) A block of titanium (Ti) of dimensions 25.0 cm x 15.0 mm x 1.00 dm has a mass of 1.69 kg. Determine the density of titanium in (i) g cm‐3 and (ii) kg m‐3. b) Look up the density of titanium and compare your answer. dexp Ti _________g cm‐3 ‐3
dexp Ti _________kg m ‐3
dlit Ti _________g cm Experiment 1 13
3. You are asked to identify a sample of an organic liquid by determining its density. You determine that 15.2 cm3 of the liquid has a mass of 43.932 g. Is it (i) bromomethane (CH3Br, d = 2.49 g cm‐3) (ii) methylene bromide (CH2Br2, d = 2.89 g cm‐3), or (iii) bromoform (CHBr3, d = 3.42 g cm‐3) ? (d = 2.89 g cm‐3, methylene bromide CH2Br2) 4. A student determines the density of Zn as 7.56 g cm‐3. Calculate the % error of this value. (5.9%) Experiment 1 14
Experiment 1: Introductory laboratory techniques: Determination of densities of solids and liquids Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS (to be entered in pen; all calculations should be shown, and should be quoted to the correct number of significant figures) Exercise in weighing Density of water (lab noticeboard) Mass of beaker/g Mass of beaker + water/g (10 cm3 measuring cylinder) ______________ ______________ ______________ Mass of water/g (10 cm3 measuring cylinder) ______________ Calculated volume of water/cm3 (10 cm3 measuring cylinder) Experiment 1 15
Mass of beaker/g Mass of beaker + water/g (50 cm3 measuring cylinder) ______________ ______________ Mass of water/g (50 cm3 measuring cylinder) ______________ Calculated volume of water/cm3 (50 cm3 measuring cylinder) Are the calculated volumes different in each case? Why might the calculated volumes be different? Which measuring device is more accurate? Densities of Liquids Liquid B Mass of measuring cylinder + liquid/g ______________ ______________ Mass of dry measuring cylinder/g ______________ ______________ Mass of liquid/g Liquid A ______________ ______________ ______________ ______________ ______________ ______________ ______________ Volume of liquid/cm3 d calculated/g cm‐3 Which is the diet soft drink? Experiment 1 16
Densities of Solids Solid C (zinc) Solid D (lead) ______________ ______________ Final volume/cm3 ______________ ______________ Initial volume/cm3 ______________ ______________ ______________ ______________ ______________ ______________ d reference value /g cm‐3 (from textbook) ______________ ______________ ______________ Mass of solid/g Volume of solid/cm3 d calculated/g cm‐3 Percentage error/% ______________ How would you modify the procedure above if you wished to determine the density of a solid such as sugar, which is soluble in water? Experiment 2 17
Experiment 2: Preparation of copper metal from copper sulfate pentahydrate AIM To prepare copper metal from copper sulfate pentahydrate by a series of reactions in order to illustrate the concepts of stoichiometry and percentage yield. INTRODUCTION In this experiment, a series of reactions will be carried out to convert copper (II) sulfate pentahydrate to copper. copper (II) sulfate → copper (II) phosphate → copper (II) chloride → copper The percentage yield will be calculated for the overall series of reactions. EXPERIMENTAL PROCEDURE Place a clean, dry 250 cm3 beaker on the balance and record the mass of the beaker. Remove the beaker from the balance, and place around 5 g of copper sulfate pentahydrate into it. Reweigh the beaker containing the copper sulphate pentahydrate. Subtracting the mass of the beaker from the mass of the beaker + copper sulfate pentahdrate will give you the mass of copper sulfate pentahydrate in the beaker. Ensure that you always use the same balance for your measurements. Weighing like this is called weighing by difference. Add 30 cm3 distilled water to the beaker and stir until the copper sulfate has dissolved completely. Now add 15 cm3 of 1 M trisodium phosphate while stirring the solution thoroughly. A light blue precipitate of copper (II) phosphate is formed. Test the solution with litmus paper. If it is acidic add 6 M NaOH dropwise with constant stirring, until it is barely basic. Add 20 cm3 distilled water, stir well and filter with a Buchner funnel. Rinse the beaker three times with small quantities of distilled water (10 cm3). Pour the washings into the filter. Finally wash the precipitate three times with 5 cm3 volumes of distilled water. Discard the filtrate. Place a clean 250 cm3 beaker under the filter funnel containing the copper (II) phosphate. Add 20 cm3 6 M HCl to the precipitate on the filter. The acid should be dropped onto the precipitate. The precipitate will dissolve and the solution will run into the beaker. This solution should be poured over the precipitate on the filter until no solid remains, replacing the collecting beaker with a clean one. When no precipitate remains, rinse the empty beaker with 1‐2 cm3 distilled water, pouring the rinse through the filter. Then rinse the filter with 1‐2 cm3 distilled water. Experiment 2 18
Clean a piece of magnesium ribbon using sandpaper. Add about 1.0 g of the clean magnesium ribbon to the solution in the beaker. Keep the magnesium submerged by using a glass rod. As the copper in solution is replaced by magnesium, the blue colour will gradually disappear. The magnesium will also react with HCl to liberate hydrogen. Stir the solution to free the copper from the magnesium metal surface. If necessary, more magnesium can be added, but care must be taken not to add too much. Should the magnesium not react, and the solution remain blue, a few drops of concentrated hydrochloric acid can be added. When the blue colour has disappeared and the magnesium is completely dissolved, a deposit of red copper will remain in a colourless solution. Allow the copper to settle, then decant the major part of the supernatant liquid and wash the copper four times by decantation with 10 cm3 portions of distilled water, taking care not to lose any solid. Drain as much water as possible from the beaker. Write your name on the beaker with a marker pen and place the beaker in the oven to drive off all moisture. When the copper is dry remove the beaker from the oven and allow it to cool. When cool, weigh the beaker and its contents. Remove all the copper from the beaker and reweigh the beaker. Calculate the mass of copper obtained. Experiment 2 19
Experiment 2: Preparation of copper metal from copper sulfate pentahydrate PRE‐LABORATORY EXERCISE 1. What is the formula of each of the following compounds? copper (II) sulfate pentahydrate ____________ copper (II) phosphate ____________ copper (II) chloride ____________ 2. Write balanced equations for the following reactions: the preparation of copper (II) phosphate from copper (II) chloride pentahydrate ________________________________________________________________________ the preparation of copper (II) chloride from copper (II) phosphate and hydrochloric acid ________________________________________________________________________ the displacement of copper from copper (II) chloride by magnesium metal ________________________________________________________________________ . 3. A student carried out Experiment 2, starting with 5.37 g copper sulfate pentahydrate. The amount of copper obtained from the experiment was 1.24 g. Calculate the percentage yield for the student’s experiment. Experiment 2 20
Experiment 2: Preparation of copper metal from copper sulfate pentahydrate Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS (to be entered in ink with all calculations shown; remember significant figures) Mass of beaker ____________ Mass of beaker + copper sulfate pentahydrate ____________ Mass of copper sulfate pentahydrate ____________ Mass of beaker + copper ____________ Mass of beaker ____________ Mass of copper obtained Percentage yield of copper ____________ ____________ Experiment 3 21
Experiment 3: An introduction to volumetric analysis: Determination of the concentration of a NaOH solution by titration with a standard solution of HCl AIM To introduce volumetric analysis. To provide practice in the correct use of the pipette and the burette, and to provide practice in the observation of indicator colour changes. To determine the concentration of an NaOH solution. INTRODUCTION In this titration you are going to determine the concentration of a NaOH solution of unknown concentration by using a standard HCl solution, the concentration of which will be given to you. You will use phenolphthalein as the indicator. A known volume of the HCl solution will be placed in the titration flask and will be titrated with the NaOH solution until the end point is reached. Phenolphthalein indicator undergoes a colour change from colourless to pink over the pH range 8.3 – 10.0. The end point is reached when a pale pink colour is observed. The reaction between NaOH and HCl is given by the following equations: NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) OH‐(aq) + H+(aq) → H2O(l) The reaction that occurs is between OH‐(aq) and H+(aq) to form unionised H2O(l). The Na+(aq) and Cl‐(aq) ions do not take part in the reaction, and are known as spectator ions. The equations above show that the OH‐(aq) and H+(aq) react in a 1:1 ratio, i.e. 1 mole of NaOH will be exactly neutralised by 1 mole of HCl. An accurate endpoint is one in which the addition of one single drop of acid or base results in the colour change that signals that the endpoint has been reached. Titrations would take far too long, however, if this principle was applied right from the beginning of every analysis; it is necessary to add single drops only when the endpoint has almost been reached. If an estimate of where the endpoint is likely to occur can be made, then acid or base can be added very rapidly (literally “run in” from the burette) until just before the endpoint is reached, and only the last 1.0 cm3 need be added drop by drop. The first titration is thus used to determine the endpoint to within the nearest 0.5 cm3, after which further accurate determinations can then rapidly be carried out. Experiment 3 22
EXPERIMENTAL PROCEDURE Introductory exercises Read appendix 3 for a description of how to use the pipette and burette correctly. The burette Pick up the burette, and turn the tap several times to get the feel of it. Put about 30 cm3 of tap water into the burette, and practice the technique of removing air bubbles trapped in the jet. Clamp the burette in the burette stand and practice opening and closing the tap with the left hand until you can control the delivery of one drop. Weigh a beaker. Now titrate exactly 25 cm3 of water into the beaker. Reweigh. This volume of water should weigh between 24.95 g and 25.05 g. If the mass of your volume is outside the range 24.90 g to 25.10 g repeat the exercise until you can achieve the desired accuracy. The pipette Pick up the pipette, one hand above the bulb, the other below the bulb. Note the graduation mark. Attach the pipette filler to the top of the pipette, and practice filling the pipette with water. Weigh three beakers, and then pipette 25 cm3 of water into each and reweigh. The mass of water in each case should be between 24.45 g and 25.05 g. Repeat this exercise until you can achieve this level of precision. Remember to keep the pipette safely lodged in the clamp when not in use. Titration of base against acid Rinse and prepare the pipette (see Appendix 3). Pipette four aliquots of 0.1 M HCl into conical flasks. Add three drops of phenolphthalein indicator to each of the four aliquots of acid. Rinse and prepare the burette (Appendix 3), then carry out a rough titration as follows: Adjust the burette level to within 0.5 cm3 of the zero mark and record the reading to the nearest 0.5 cm3. It is not necessary to take accurate readings at this stage, this is only a rough titration. Run in about 15 cm3 of NaOH solution from the burette whilst swirling the contents of the conical flask. Thereafter add ~0.5 cm3 at a time until the colour change from colourless to pink signals the endpoint has been passed, and again record this value to within 0.5 cm3. Experiment 3 23
You now have a rough idea of where the titration endpoint occurs, and as the other three aliquots were prepared in the same way, the endpoints for these should also (allowing for experimental error) be the same. Refill the burette and take an accurate initial reading, this time to the nearest 0.02 cm3. Run in NaOH solution to within 1.0 cm3 of the rough endpoint determined above, and dropwise from then on. Keep swirling the conical flask throughout the titration, and rinse the inside of the flask down frequently with deionised water from your washbottle. As the endpoint approaches, the pink colour that appears when a drop of NaOH solution is added will take longer and longer to disappear, and a close watch must be kept on the colour of the solution after the addition of a drop and after swirling. Eventually, the addition of one drop will change the colour of the solution. When the faint pink colour that appears persists for ~15 seconds, the endpoint has been reached. Read and record the final burette volume, and calculate the volume of NaOH delivered (the “endpoint volume”) before continuing with the next accurate titration. Repeat for the third and fourth aliquots, refilling the burette as required (if each titration requires less than 25 cm3, two titrations can be performed before refilling). NOTE: It is all too easy for beginners to add too much NaOH and go past, or overshoot the endpoint. Obviously these results will be incorrect. Record them anyway ‐ they will be discarded later, but it is very important to develop the habit of recording all results, not just the good ones. After a little practice, the intermediate colour should be attained in every titration, especially when the technique of adding a fraction of a drop is mastered. If you do not obtain three titrations within 0.10 cm3, repeat the procedure until you do. Select the three titrations in closest agreement (within 0.10 cm3) and calculate their average. Calculate the exact molarity of the approximately 0.1 M NaOH solution from the exact molarity of the HCl solution given to you and your titration results. Remember significant figures. Now perform a further four titrations, two with methyl red as the indicator and two with screened methyl orange as the indicator. In each case record your results and note the colour changes observed. CLEANING UP Rinse the pipette with deionised water and lodge it in the clamp upside down. Rinse the burette with deionised water, and lodge it in the clamp upside down with the stopcock in the open position. Experiment 3 24
Experiment 3: An introduction to volumetric analysis: Determination of the concentration of a NaOH solution by titration with a standard solution of HCl PRE‐LABORATORY EXERCISE A 0.1056 M solution of HCl was titrated with a solution of NaOH of unknown concentration. When a 20.00 cm3 aliquot of the HCl was used, the following results were obtained: Titration No. 1
2
3
4 5 3
20.64
40.62
20.70
41.08 20.88
Final burette reading/cm 3
0.52
20.74
0.62
21.12 0.78
Initial burette reading/cm Volume delivered /cm3 Identify the three acceptable results, and use them to calculate the exact molarity of the NaOH solution. Also calculate the NaOH concentration in g dm‐3 (molar mass of NaOH = 40.00 g mol‐1). (acceptable results 20.12, 20.08, 20.10 cm3 (range ≤ 0.10 cm3)) (average 20.10 cm3, [NaOH] 0.1051 M, 4.203 g dm‐3) Experiment 3 25
Experiment 3: An introduction to volumetric analysis: Determination of the concentration of a NaOH solution by titration with a standard solution of HCl Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Preliminary exercises Masses for 25 cm3 of water/g Burette Pipette Experimental Titration Results (ALL BURETTE READINGS MUST BE GIVEN TO 2 DECIMAL PLACES.) HCl molarity ____________M Volume of HCl aliquots used ____________cm3 Titration No. Rough 1 2
3
Final burette 3
reading/cm Initial burette 3
reading/cm Volume 3
delivered/cm Circle the titration numbers used for the calculations. 4
5 6 7
Experiment 3 Average volume delivered ____________cm3 Balanced equation for the titration reaction: Molarity of NaOH in mol dm‐3 ____________M Concentration of NaOH in g dm‐3 (MM NaOH 40.00 g mol‐1) ____________g dm‐3 NaOH 26
Experiment 4 27
Experiment 4: Determination of carbonate in an antacid tablet by titration against a standardised acid solution AIM To provide further practice in titration technique, and to introduce the concept of analysing a substance to determine the percentage of a particular chemical it contains. INTRODUCTION Antacids, such as Rennies, are products used to relieve heartburn. Heartburn is caused by stomach acid (~ 0.02 M HCl) refluxing up into the oesophagus. Antacids increase the pH of this acid to about pH 3 or 4, relieving the pain of heartburn. Most antacids contain neutralising agents such as calcium carbonate, sodium bicarbonate, magnesium hydroxide or aluminium hydroxide. They also contain other ingredients to give flavour and hold the tablet together. In this experiment, the percentage carbonate in a commercial antacid tablet will be determined by titrimetric analysis. The carbonate anion readily reacts with acid, liberating gaseous carbon dioxide: CO32‐(aq) + 2H3O+(aq) → 2H2O(l) + CO2(g) It is thus possible to determine the concentration of carbonate in solution by titration with an acid solution of known concentration. In this titration HCl(aq) is used: Na2CO3(aq) + 2HCl(aq) → 2H2O(l) + 2NaCl(aq) + CO2(g) so the stoichiometry is 1:2. The indicator used is screened methyl orange, which undergoes a colour change from bright green in basic medium, through a grey‐green endpoint, to purple in acid. The exact position of the endpoint in this titration is affected by the CO2 remaining in solution. To obtain an accurate endpoint, the solution is boiled to remove the dissolved CO2. It is best to do this immediately before the endpoint is reached. Each sample is thus titrated to the intermediate, or “cold” endpoint, at which point the solution should be grey‐green, and then boiled to remove the dissolved CO2. As CO2 dissolves in H2O to give carbonic acid, H2CO3(aq), this procedure effectively makes the solution more basic, and the solution “retreats” from the endpoint, becoming slightly more green. The solution is then titrated further to the final, or “hot” endpoint, which is the accurate value. Experiment 4 28
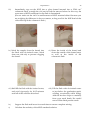
Each titration will be carried out on part of a commercial antacid tablet. As the mass of each sample is different, the endpoint of each titration will also be different. A rough endpoint can be calculated for each sample, based on the mass weighed out, an HCl molarity of 1.0 M, and the percentage carbonate stated in the ingredients list of the product to be analysed. This will be a rough value – the true endpoint may be higher or lower. EXPERIMENTAL PROCEDURE Rinse and prepare a burette with ~0.2 M HCl solution. Also clean and rinse four conical flasks with deionised water. Weigh an antacid tablet by difference (see experiment 2). Crush the tablet in a beaker with a glass rod. Weigh out three ~0.25 g samples of the crushed tablet into conical flasks. Record the masses. Dissolve each sample in ~25 cm3 of deionised water and add 3 drops of screened methyl orange indicator. Each sample in turn is then treated as follows: Calculate the rough endpoint volume as explained above, and “run in” to within 1 cm3 of this value. Continue titrating dropwise until the intermediate, or “cold”, endpoint is reached. Do not record this value, it is not the final, correct endpoint. Add three anti‐bumping granules and boil the solution for two minutes. You should observe a colour change from grey‐green (endpoint) back to green (basic) as the dissolved CO2 is driven off. The correct way to boil a solution in the laboratory is shown in the figure below. This photograph shows the correct setup for boiling a solution in the laboratory. Nothing that will get hot (or is hot) should be placed directly on the benchtop. The Bunsen burner is placed on an insulating tile. The vessel containing the liquid to be boiled should be placed on a gauze mat resting on an iron ring or a tripod. Experiment 4 29
Finally, titrate while hot to the final, correct endpoint. This should require no more than another ~0.1 ‐ 0.2 cm3. Record this value, and calculate the percentage carbonate in the sample before beginning the next titration. If you slightly overshoot the original “cold” endpoint (i.e. the solution is purple/magenta rather than grey‐green), try boiling the solution anyway. Providing the original error was not too large, this may yet produce an acceptable endpoint. Continue titrating samples until you have three with a percentage carbonate range of not more than 0.50 %. Process your results and calculate the average percentage carbonate in the tablet. To calculate percentage carbonate in each sample the following formula can be used: g carbonate by titration
% carbonate =
× 100 g sample
Percentage error can be calculated as follows: g carbonate by titration ‐ g carbonate from label
% error =
× 100 g carbonate from label
Refer to the noticeboard to obtain the information from the label, i.e. ʺg carbonate from labelʺ. 30
Experiment 4 Experiment 4: Determination of carbonate in an antacid tablet by titration against a standardised acid solution PRE‐LABORATORY EXERCISE 1. Show that a sample of antacid containing ~1.06 g Na2CO3 would require ~20 cm3 of ~1.0 M HCl to reach the endpoint. 2. In an experiment to determine the % purity of a sample of Na2CO3, a student obtains the following results. Calculate the % purity of the Na2CO3 sample. Sample 1
Molarity HCl/M 1.024 Mass of weighing vial + Na2CO3 sample/g 10.454 Mass of weighing vial after emptying/g 9.332 Mass of Na2CO3 sample/g Calculated minimum endpoint (± 0.5 cm3) 18.5 Final (hot) burette reading/cm3 19.88 Initial burette reading/cm3 0.92 Volume delivered/cm3 n HCl at endpoint/mol n Na2CO3 in sample/mol Mass Na2CO3 in sample/g % purity of sample Experiment 4 31
(n HCl 1.942 x 10‐2 mol, n Na2CO3 9.708 x 10‐3 mol, mass pure Na2CO3 1.029 g, 91.70%) 3. Continuing the analyses, the same student obtains further results of 90.96 %, 91.24 %, and 91.39 %. Which value should be discarded, and why? (91.70!!. 90.96, 91.24, 91.39 more precise (range 0.43%) than 91.70, 91.24, 91.39 (0.46%)) 32
Experiment 4 Experiment 4: Determination of carbonate in an antacid tablet by titration against a standardised acid solution Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Molarity of HCl ______________ M Mass of tablet ______________ g % carbonate from ingredients list (lab noticeboard) ______________g Sample Mass of conical flask/g 1 2 3 4 5 Mass of conical flask + tablet sample/g Mass of antacid sample/g Calculated rough endpoint (± 0.5 cm3) Final (“hot”) burette reading/cm3 Initial burette reading/cm3 Volume delivered/cm3 n HCl at endpoint/mol n Na2CO3 in sample/mol Mass Na2CO3 in sample/g % purity of sample CIRCLE the values used in calculations, and calculate the average % purity of the sample. Experiment 4 Show all your calculations, and enter your answers into the table above. Average % purity ____________% 33
Experiment 5 34
Experiment 5: Preparation of a primary standard solution of potassium hydrogen phthalate and its use in determining the concentration of a basic solution AIM To prepare a standard solution of known concentration from a primary standard acidic substance, potassium hydrogen phthalate (KHP), and to use this standard solution to accurately determine the concentration of a sodium hydroxide solution by titration. INTRODUCTION A primary standard is a solution for which the concentration is accurately known, that can be used to determine the concentration of solutions of unknown concentration. A limited number of substances can be used as primary standards in volumetric analysis. The material must be available in high purity (e.g. >99.99 %), it must be stable and non‐
hygroscopic and it should react with other substances quantitatively and in known proportions. The salt potassium hydrogen phthalate, (KOOCC6H4COOH, molar mass 204.2 g mol‐1, abbreviated as KHP) meets these requirements and is commonly used to prepare a standard acidic solution for use in acid‐base titrations. H
CO2H
CO2K
H
CO2H
CO2H
H
phthalic acid
C
C
C
C
O
C
C
C
C
H
O
potassium hydrogen phthalate
OK
O
H
KHP is the monopotassium salt of the diprotic acid phthalic acid. It thus reacts with NaOH as a monoprotic acid according to the equation: KOOCC6H4COOH(aq) + NaOH(aq) → KOOCC6H4COONa(aq) + HOH(l) Thus the stoichiometry of the reaction is 1:1, and at the endpoint of the titration an equal number of moles of potassium hydrogen phthalate and sodium hydroxide will have reacted. As the mass and molar mass of KHP, and the volume of the solution made up, are all known, the molarity of the KHP solution can be accurately calculated. Consequently, the moles of KHP used in the titration are known (from the molarity of the solution and the volume of the aliquot used in the titration), and the concentration of sodium hydroxide in a solution can be determined accurately by titration. Experiment 5 35
Calculation of the mass of KHP required We wish to make up 250 cm3 of a 0.1 M primary standard solution. The mass required can be calculated as follows: mass required = moles (n) x molar mass (MM) = {molarity (mol dm‐3) x volume (dm3)} x MM = ~0.1 mol dm‐3 x 0.250 dm3 x 204.2 g mol‐1 Thus a mass of 5.1 g is required. In practice, any mass between 4.5 and 5.5 g is satisfactory, provided it is weighed accurately. EXPERIMENTAL PROCEDURE Rinse and prepare a burette with ~0.1 M NaOH solution. Also clean and rinse (with deionised water) four conical flasks, a 250.0 cm3 volumetric flask, a glass funnel, and a sample vial. The sample vial must be dry before placing on the balance. Preparation of the standard solution i) Tare (place on the balance and zero) a clean dry sample vial, then remove from the balance, and add 5.2‐5.5 g of KHP. (A demonstration sample vial will be available at the balance to give you a rough idea of how much to add; at no stage must KHP be added to the vial while it is on the balance.) ii) Once the correct rough mass has been added, remove the vial from the balance, zero the balance, and accurately weigh the vial and contents. Record the mass. Experiment 5 iii) 36
Immediately tap out the KHP into a glass funnel inserted into a 250.0 cm3 volumetric flask, reweigh the vial, and record the mass as before. In this way, the exact mass of KHP transferred to the flask is determined. (Do not wash out the vial if a small amount of KHP remains behind: because you are weighing by difference it does not matter, as long as all of the KHP that left the vial ended up in the volumetric flask.) iv) Wash the sample from the funnel into the flask with de‐ionised water. Wash from one side only to prevent clogging the funnel. vi) Rinse the inside of the funnel and down the outside of the funnel stem, as well as the inside of the volumetric flask. vii) Half‐fill the flask with de‐ionised water, and swirl vigorously for 10‐15 minutes until all of the solid has dissolved. viii) Fill the flask with de‐ionised water to just below the graduation mark, swirling occasionally while filling. Add the last few drops or carefully from your wash bottle. Be careful not to fill the flask past the mark. ix) x) Stopper the flask and invert it several times to ensure complete mixing. Calculate the molarity of the KHP standard solution. Experiment 5 37
Carrying out the titration From the molarity of the KHP solution calculated above, calculate the approximate (to the nearest 0.5 cm3) volume of 0.1 M NaOH solution required to reach the endpoint when titrating 25 cm3 standard KHP solution. Pipette 25.00 cm3 aliquots of primary standard acid solution into each of four Erlenmeyer flasks. Add 4 drops of phenolphthalein indicator. Use the first aliquot as a rough titration, to confirm the value calculated above. Titrate the remaining three aliquots in turn until the solution just turns pink, which corresponds to the endpoint. Record each volume to ±0.02 cm3. Continue titrating aliquots until you obtain three endpoint volumes within a 0.10 cm3 range. Average the three volumes and calculate the concentration of the NaOH solution in mol dm‐3 and g dm‐3. Experiment 5 38
Experiment 5: Preparation of a primary standard solution of potassium hydrogen phthalate and its use in determining the concentration of a basic solution PRE‐LABORATORY EXERCISE The labels on chemical bottles sometimes deteriorate if the bottles are left for a long time in a chemical store. One such bottle containing a solution can be identified only as a NaOH solution, as the label indicating its concentration has fallen off. Use the results below to calculate the molarity of the NaOH solution, and its concentration in g dm‐3. (MM NaOH 40.00 g mol‐1) Mass of weighing vial + KHP/g 14.563 Mass of weighing vial after emptying/g 9.301 Mass of KHP/g Molarity of KHP solution/mol dm‐3 Experiment 5 39
Titration No. 1
2
3
4 Final burette reading/cm3 19.24 38.34 19.66 38.54 Initial burette reading/cm3 0.26 19.44 0.58 19.66 Volume delivered/cm3 _______________________________ Concentration of NaOH in g dm‐3: ([KHP] 0.1031 M, average volume 18.92 cm3, [NaOH] 0.1090 M, 4.358 g dm‐3) Experiment 5 40
Experiment 5: Preparation of a primary standard solution of KHP and its use in determining the concentration of a basic solution Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Preparation of KHP standard solution Mass of weighing vial + KHP: ___________ g Mass of weighing vial after emptying: ___________ g Mass of KHP: ___________ g ___________ cm3 Molarity of KHP solution: ___________ mol dm‐3 Calculated rough endpoint: ___________ cm3 Volume of volumetric flask: Experiment 5 41
Experimental Titration Results (All burette readings must be given to 2 decimal places) Titration No. Rough 1 2
3
4
5 6 Final burette reading/cm3 Initial burette 3
reading/cm Volume 3
delivered/cm Circle the titration numbers used in the calculations. Average volume delivered ____________cm3 Balanced equation for the titration reaction: Molarity of NaOH in mol dm‐3 and concentration in g dm‐3 ____________M ____________g dm‐3 7
Experiment 6 42
Experiment 6: Determination of the concentration of acetic acid, CH3COOH, in a sample of vinegar AIM To prepare a KHP primary standard and to use this to standardise a NaOH solution. To use the standardised NaOH to determine the concentration of acetic acid in a sample of vinegar, and hence to calculate the grams of acetic acid in 100 cm3 of sample. INTRODUCTION In this experiment a KHP primary standard will be prepared following the same procedure as in Experiment 5. This primary standard solution will be used to standardise a solution of NaOH, as before. The NaOH solution will be used to determine the concentration of acetic acid in a sample of vinegar. Hence this concentration can be expressed as a percentage (m/v), where m mass of acetic acid in grams
=
v
100 cm 3 of vinegar sample
EXPERIMENTAL Preparation of Standard KHP Solution (see page 36) Weigh about 2 g of KHP into a sample vial. Determine the exact mass of the KHP and vial. Pour the KHP from the vial into a funnel placed in a 250 cm3 volumetric flask. Reweigh the empty vial and hence determine the exact mass of KHP transferred to the flask. Wash the sample into the volumetric flask with deionised water, rinse the funnel appropriately, and add water until the flask is about half full. Swirl the flask vigorously to dissolve the KHP totally. Carefully add water until the calibration mark is reached exactly. Stopper the flask and mix the solution thoroughly by inverting the flask several times. Calculate the molarity of the standard KHP solution that you have prepared. Standardisation of NaOH solution Rinse and prepare a burette with ~0.1 M NaOH solution. From the molarity of the KHP solution calculated above, calculate the approximate (to the nearest 0.5 cm3) volume of 0.1 M NaOH solution required to reach the endpoint when titrating 25 cm3 standard KHP solution. Experiment 6 43
Pipette 25.00 cm3 aliquots of primary standard acid solution into each of four clean Erlenmeyer flasks. Add 4 drops of phenolphthalein indicator. Use the first aliquot as a rough titration, to confirm the value calculated above. Titrate the remaining three aliquots in turn until the solution just turns pink, which corresponds to the endpoint. Record each volume to ±0.02 cm3. Continue titrating aliquots until you obtain three endpoint volumes within a 0.10 cm3 range. Average the three volumes and calculate the concentration of the NaOH solution in mol dm‐3 and g dm‐3. Determination of the concentration of acetic acid in the vinegar sample Top up your burette with NaOH and carry out a series of titrations as above, using 25.00 cm3 aliquots of the vinegar sample instead of KHP. Continue titrating aliquots until you obtain three endpoint volumes within a 0.10 cm3 range. Use your titration results to determine the concentration of acetic acid in the vinegar. Hence calculate the grams of acetic acid per 100 cm3 of vinegar sample (or % (m/v)). Experiment 6 44
Experiment 6: Determination of the concentration of acetic acid, CH3COOH, in a sample of vinegar PRE‐LABORATORY EXERCISE 1. Write a balanced equation for the reaction between acetic acid and NaOH. 2. A student carried out a similar experiment to that described in Experiment 6 and obtained the data given in the table below. Calculate the molarity and % (m/v) of the acetic acid in the vinegar sample. The molarity of the standardised NaOH solution was 0.1126 mol dm‐3. Titration No.
Rough
1
2
3 4 Final burette reading/cm3 24.24 24.22 48.32 24.42 48.44 Initial burette reading/cm3 0.24 0.26 24.32 0.58 24.52 Volume delivered/cm3 (0.1079 M, 0.6479 % (m/v)) Experiment 6 45
Experiment 6: Determination of the concentration of acetic acid, CH3COOH, in a sample of vinegar Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Preparation of KHP standard solution Mass of weighing vial + KHP: ___________ g Mass of weighing vial after emptying: ___________ g Mass of KHP: ___________ g ___________ cm3 Molarity of KHP solution: ___________ mol dm‐3 Calculated rough endpoint: ___________ cm3 Volume of volumetric flask: Experiment 6 46
Experimental Titration Results (All burette readings must be given to 2 decimal places) Titration No. Rough 1 2
3
4
5 6 Final burette reading/cm3 Initial burette 3
reading/cm Volume 3
delivered/cm Circle the titration numbers used in the calculations. Average volume delivered ____________ cm3 Balanced equation for the titration reaction: Molarity of NaOH in mol dm‐3 and concentration in g dm‐3 ____________M ____________g dm‐3 7
Experiment 6 47
Determination of the concentration of acetic acid in the vinegar sample Titration of vinegar sample with standard NaOH solution Titration No. Rough 1 2
3
Final burette 3
reading/cm Initial burette reading/cm3 Volume 3
delivered/cm Circle the titration numbers used in the calculations. Average volume delivered ____________ cm3 Balanced equation for the titration reaction: Molarity and % (m/v) of CH3COOH in vinegar sample: ____________M 4
5 6 7
____________% (m/v) Experiment 7 48
Experiment 7: Determination of the concentration of a solution of an ammonium salt by means of a back titration AIM To introduce the concept and technique of back titration, and to determine the concentration of a solution of an ammonium salt. INTRODUCTION An ammonium salt solution will react with sodium hydroxide, liberating ammonia from the solution according to the following reaction: NH4X(aq)+ NaOH(aq) → NH3(g) + H2O(l) + NaX(aq). If a known excess of sodium hydroxide is added to the ammonium salt solution, then the sodium hydroxide remaining after the above reaction has occurred can be neutralised by titration with a standard acid such as HCl: NaOH + HCl → NaCl + H2O. This is called a back titration because the difference in the amount of sodium hydroxide originally added and that neutralised by the acid can be used to determine the concentration of the ammonium salt solution. In this experiment the ammonium salt is ammonium chloride, NH4Cl. NH4Cl and NaOH react only slowly at room temperature, so the reaction mixture has to be boiled to make the two compounds react completely. The indicator used is methyl red, which undergoes a colour change from yellow in basic medium, through orange at about pH 5, to red in acid. EXPERIMENTAL You are provided with a solution of NH4Cl of unknown concentration, and standardised NaOH (~ 0.2 M) and HCl (~ 0.1 M) solutions. Set up four 250 cm3 Erlenmeyer flasks. Into each pipette 25 cm3 of the ammonium salt solution. Then add, by pipette, 25 cm3 of the sodium hydroxide solution. Add two anti‐
bumping granules to each flask. Experiment 7 49
Treat each sample in turn as follows: Place a small funnel in the neck of the flask to prevent loss of liquid (due to splashing) while boiling the contents. Boil the mixture (see page 29) until a piece of moistened red litmus paper held in the escaping steam no longer turns blue. This will take between 10 and 20 minutes. Add water as necessary – the solution volume should be kept at approximately 40 cm3. On no account allow the solution to evaporate to dryness. Cool the solution under a stream of cold water, add a few drops of methyl red indicator and titrate the remaining sodium hydroxide with the standard 0.1 M HCl. Use the first flask as a rough titration. Continue titrating until 3 results agree within 0.10 cm3. Calculate the concentration of the NH4Cl solution (molar mass NH4Cl = 53.49 g mol‐1). Experiment 7 50
Experiment 7: Determination of the concentration of a solution of an ammonium salt by back titration PRE‐LABORATORY EXERCISE 1. Using 20.00 cm3 aliquots each of NH4Cl and standard NaOH solution (0.2409 M) in an experiment to determine the molarity of an NH4Cl solution, a student obtains the following results. Calculate the molarity of the NH4Cl solution. Molarity HCl 0.1027 M Final burette reading 19.68 cm3 Initial burette reading 0.34 cm3 Volume HCl delivered _____________ cm3 moles NaOH pipetted originally _____________ mol _____________ mol moles HCl at endpoint n NaOH that reacted with NH4Cl solution _____________ mol moles NH4Cl present in original NH4Cl solution _____________ mol Experiment 7 51
Molarity NH4Cl _____________ M Concentration NH4Cl _____________ g dm‐3 ([NH4Cl] 0.1056M, 5.649 g dm‐3) 2. Why: a) must the NH4Cl/NaOH mixture be heated before titrating to the endpoint with HCl? b) should the solution volumes be kept at 40 cm3? (hint: endpoint colour) Experiment 7 52
Experiment 7: Determination of the concentration of a solution of an ammonium salt by back titration Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Molarity of HCl ______________M Molarity of NaOH ______________M Experimental Titration Results Titration No. Rough 1 Final burette 3
reading/cm Initial burette reading/cm3 Volume 3
delivered/cm 2
3
5 6 7
Circle the titration numbers used in the calculations. Balanced equation for the titration reaction: Average volume delivered _______cm3 4
Experiment 7 Concentration of the NH4Cl solution (MM NH4Cl 53.49 g mol‐1) ____________M NH4Cl ____________g dm‐3 NH4Cl 53
Experiment 8 54
Experiment 8: Determination of the percentage purity of a sample of Mohr’s salt by redox titration with the permanganate ion, MnO4G AIM To introduce the concept and technique of a redox titration, and to determine the percentage purity of an iron‐containing salt. INTRODUCTION Iron has two common oxidation states, Fe2+ and Fe3+. Fe2+ can be easily and quantitatively oxidised to Fe3+. A suitable oxidising agent is permanganate, MnO4G, which in acid medium yields Mn2+ on reduction. Fe2+(aq) → Fe3+(aq) + e‐ G
MnO4 (aq) + 8H+(aq) +5e‐ → Mn2+(aq) + 4H2O(l) Note that each MnO4− ion requires 5 electrons, thus 1 MnO4− ion is equivalent to 5 Fe2+ ions, or G
2+
+
‐
MnO4 (aq) + 5Fe (aq) + 8H (aq) → Mn2+(aq) + Fe3+(aq) + 4H2O(l) In this experiment the percentage purity of a soluble iron‐containing salt is determined by dissolving a sample and titrating the Fe2+(aq) present with a solution of MnO4G(aq) of known concentration. The salt in this exercise is Mohr’s salt, FeSO4(NH4)2SO4.6H2O. It is water‐soluble, with a mass of ~1 g containing ~2.5 mmol Fe2+, and requiring ~20 cm3 of ~0.025 M KMnO4 solution for complete reaction. KMnO4 is used as the oxidising agent as it is “self‐indicating” ‐ the purple colour of the MnO4G(aq) disappears as it is added to the salt solution, because reaction with the Fe2+(aq) converts it to colourless Mn2+(aq). Once the Fe2+(aq) has all reacted, however, the first drop of excess MnO4G(aq) added gives a pale pink endpoint. As the mass of each sample in this experiment is different, the endpoint of each titration will also be different. A rough endpoint can be calculated for each sample, based on the mass of salt weighed out, the percentage by mass of Fe in Mohr’s salt (calculated in the pre‐laboratory exercise), and the molarity of the KMnO4. Experiment 8 55
EXPERIMENTAL Rinse and prepare a burette with ~0.025 M KMnO4 before weighing out the samples of Mohr’s salt for analysis. Also clean and rinse four conical flasks and two pill vials. Accurately weigh out by difference four samples of 1.100‐1.200 g of Mohr’s salt. No sample should have a mass of <1.000 g. To each sample add 10 cm3 3 M H2SO4, 50 cm3 of deionised water and 5 cm3 conc. phosphoric acid (H3PO4). Swirl until the Mohr’s salt is dissolved. Each sample in turn is then treated as follows: Calculate the minimum endpoint volume as explained above, and “run in” to ~0.5 ‐ 1 cm3 below this value, then continue adding dropwise until the endpoint is reached. Remember to wash down the sides of the flask with deionised water while doing this. Record the volume delivered, and calculate the percentage purity of the sample before beginning the next titration. Continue titrating samples until you have three with a percentage purity range of not more than 0.25 %. Experiment 8 56
Experiment 8: Determination of the percentage purity of a sample of Mohr’s salt by redox titration with the permanganate ion, MnO4G PRE‐LABORATORY EXERCISE 1. Determine the molar mass of Mohr’s salt, FeSO4(NH4)2SO4.6H2O, and the % by mass of iron in this salt. (392.19 g mol‐1, 14.24%) 2. A student carried out Experiment 8 and obtained the following results. Calculate the percentage purity of the student’s sample. Molarity KMnO 4 0.02596 M Mass of weighing vial + salt sample 10.454 g Mass of weighing vial after emptying 9.288 g Mass of salt sample ___________ g Calculated minimum endpoint (± 0.5 cm3) ___________ cm3 Experiment 8 Final burette reading Initial burette reading Volume MnO4G solution delivered 57
23.66 cm3 0.92 cm3 ___________ cm3 ___________ mol ___________ mol ___________ g ___________ ___________ moles MnO4G at endpoint moles Fe2+ in sample Mass Fe2+ in sample % Fe2+ in sample % purity of sample (n MnO4G 5.903 x 10‐4 mol, n Fe2+ 2.952 x 10‐3 mol, mass Fe2+ 0.1648 g, 14.14%) Experiment 8 58
Experiment 8: Determination of the percentage purity of a sample of Mohr’s salt by redox titration with the permanganate ion, MnO4G Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Molarity of KMnO4 ______________ M Sample 1
2
3
4 5
Mass of weighing vial + salt sample/g Mass of weighing vial after emptying/g Mass of salt sample/g Calculated minimum endpoint (± 0.5 cm3) Final burette reading/cm3 Initial burette reading/cm3 Volume delivered/cm3 n MnO4G at endpoint/mol n Fe2+ in sample/mol Mass Fe2+ in sample/g % Fe2+ sample Balanced equation for the titration: Experiment 8 59
Circle the titration numbers used in the calculations. Show all calculations below, and fill in the answers in the table overleaf. Calculate the average % iron in your sample, and hence the percentage purity of your sample. Average % iron ____________% Percentage purity ____________%
Experiment 9 Experiment 9: 60
Determination of the molar mass of magnesium by the eudiometric method AIM To determine the molar mass of magnesium by displacement of hydrogen from an acid. INTRODUCTION Reactive metals, such as magnesium, will react with an acid to form a salt and hydrogen gas: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g). The equation above shows that 1 mole of magnesium will evolve 1 mole of hydrogen gas. The molar mass of the metal can be determined by measuring the volume of H2 produced by reaction of a metal sample of known mass with HCl. Hydrogen will be treated as an ideal gas, i.e. 1 mole of H2 occupies 22.41 dm3 at standard temperature and pressure (STP). The apparatus used in this experiment is shown in the digram below. gas collecting
water (hydrostatic head)
eudiometer tube
water
beaker
Hirsch funnel
magnesium ribbon
With this apparatus, the volume (V), temperature (T) and pressure (p) of a small volume of hydrogen gas, liberated by a small mass (m) of magnesium, can be measured. Experiment 9 61
The pressure of the gas in the eudiometer tube is determined by: •
the pressure of the hydrogen gas itself •
the pressure of the water vapour in the hydrogen gas •
the hydrostatic head of water in the eudiometer tube (see figure) •
atmospheric pressure in the laboratory at the time of the experiment. To obtain the pressure of the hydrogen gas alone, the water vapour pressure and the hydrostatic head must be subtracted from atmospheric pressure (see table). The hydrostatic head is measured in mm water, and can be converted to Pa using the following conversion factors: 1
1 mm water = mm Hg 13.6
1 mm Hg = 0.133 kPa . The volume of hydrogen gas collected can be read off from the eudiometer tube. The temperature of the gas is assumed to be the same as that of the water in the beaker. Hence the number of moles of hydrogen gas collected can be calculated from the ideal gas equation. Since the reaction taking place is 1:1 between Mg and H2 this allows one to calculate the molar mass of Mg. EXPERIMENTAL Clean a strip of magnesium ribbon with sandpaper. Do this on an insulating tile and not on the wooden laboratory bench! (Magnesium metal is reactive, so it is covered with an oxide layer formed by reaction with oxygen in the air. This must be removed to allow the magnesium to react in this experiment.) Fold the strip into a Z shape, and weigh it accurately. Fill a 2 dm3 beaker to near the top with tap water. Put the magnesium ribbon in the beaker, and cover it with a Hirsch funnel. Fill the eudiometer tube to overflowing with tap water, and slide your thumb over the end so that there are no air bubbles. Submerge the tube under the water level, and place it over the Hirsch funnel so as not to allow any gas to escape. Ensure that no air bubbles are trapped in the tube. Syphon out any excess water. Measure 30 cm3 concentrated hydrochloric acid, pour it into the beaker and stir with a glass rod. Gas evolution should start within a few minutes, and the reaction should be complete within 20 minutes. Agitate the funnel every now and then to release gas bubbles in the neck of the funnel. Read and record the volume of gas in the tube, measure and record the temperature of the water in the beaker, and measure and record the hydrostatic head. Calculate the molar mass of magnesium. Experiment 9 TABLE: VAPOUR PRESSURE OF WATER IN kPA Temp/°C 0.0
0.2
0.4
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 0.6104 0.6566 0.7057 0.7578 0.8133 0.8722 0.9348 1.0015 1.0724 1.1476 1.2275 1.3122 1.4020 1.4971 1.5979 1.7046 1.8174 1.9368 2.0631 2.1964 2.3374 2.4860 2.6429 2.8084 2.9829 3.1667 3.3604 3.5643 3.7789 4.0047 4.2421 4.4915 4.7539 5.0293 5.3305 5.6219 5.9402 6.2740 6.6239 6.9905 7.3747 0.6194 0.6662 0.7158 0.7686 0.8247 0.8844 0.9479 1.0153 1.0871 1.1633 1.2441 1.3298 1.4207 1.5168 1.6188 1.7266 1.8407 1.9615 2.0892 2.2241 2.3665 2.5167 2.6753 2.8425 3.0190 3.2044 3.4003 3.6064 3.8230 4.0513 4.2911 4.5431 4.8079 5.0861 5.3779 5.6844 6.0057 6.3427 6.6958 7.0661 7.4528 0.6285 0.6758 0.7261 0.7795 0.8363 0.8968 0.9610 1.0293 1.1020 1.1790 1.2608 1.3475 1.4395 1.5367 1.6388 1.7490 1.8645 1.9866 2.1156 2.2520 2.3959 2.5478 2.7081 2.8770 3.0555 3.2427 3.4407 3.6490 3.8677 4.0983 4.3404 4.5950 4.8624 5.1432 5.4381 5.7475 6.0717 6.4120 6.7682 7.1422 7.5328 62
0.6 0.8
0.6378 0.6857 0.7365 0.7906 0.8482 0.9094 0.9743 1.0435 1.1770 1.1950 1.2777 1.3655 1.4584 1.5569 1.6612 1.7716 1.8883 2.0118 2.1423 2.2801 2.4257 2.5792 2.7413 2.9119 3.0923 3.2814 3.4814 3.6919 3.9129 4.1459 4.3901 4.6474 4.9176 5.2011 5.4988 5.8112 6.1385 6.4820 6.8414 7.2190 7.6128 0.6472 0.6957 0.7471 0.8018 0.8602 0.9220 0.9879 1.0579 1.1323 1.2112 1.2949 1.3837 1.4776 1.5773 1.6828 1.7944 1.9125 2.0374 2.1691 2.3086 2.4557 2.6109 2.7746 2.9473 3.1294 3.3208 3.5226 3.7352 3.9586 4.1938 4.4405 4.7003 4.9732 5.2596 5.5599 5.8756 6.2059 6.5526 6.9155 7.2964 7.6941 Experiment 9 63
Experiment 9: Determination of the molar mass of magnesium by the eudiometric method PRE‐LABORATORY EXERCISE 1. Rewrite the ideal gas equation, solving it for: pressure number of moles temperature 2. If a student forgot to subtract the water vapour pressure from the atmospheric pressure in determining the number of moles of hydrogen gas produced, would the calculated amount of moles be too high or too low? Show your reasoning. 3. A 0.0785 g sample of Ca was used to produce H2(g) by the reaction Ca(s) + 2HCl(aq) → CaCl2(aq) + H2(g). The atmospheric pressure was recorded as 708.5 Torr and room temperature was 22 °C. When the water levels in the eudiometer and the beaker were equal, the volume of H2 collected measured as 50.7 cm3. (1 Torr ≡ 1 mm Hg, and 760 Torr = 101325 Pa; vapour pressure of water at 22 °C = 19.8 Torr; R = 8.315 J K‐1 mol‐1). Experiment 9 64
Calculate the number of moles of hydrogen gas produced in the reaction. Calculate the theoretical number of moles that could be obtained with the given amount of metal. Finally, determine the percentage yield of hydrogen. (n H2 = 0.0019 mol, % yield = 95 %) Experiment 9 Experiment 9: 65
Determination of the molar mass of magnesium by the eudiometric method Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Data Mass of magnesium ___________ g Temperature of the system ___________ °C Atmospheric pressure ___________ mm Hg Hydrostatic head ___________ mm water Water vapour pressure (at temperature of system) ___________ kPa Volume of hydrogen ___________ cm3 ___________ kPa ___________ K ___________ kPa Calculations Atmospheric pressure Temperature Hydrostatic correction Experiment 9 66
Corrected pressure for dry H2 ___________ kPa ___________ mol ___________ mol ___________ g mol‐1 Molar mass of Mg (theoretical) ___________ g mol‐1 ___________ % Number of moles of H2 gas Number of moles of Mg metal Molar mass of Mg (calculated) Percentage error Experiment 10a 67
Experiment 10a: The absolute zero of temperature AIM To determine the absolute zero of temperature, and to illustrate that P/T is a constant, provided T is measured in degrees Kelvin (K). INTRODUCTION When a gas is heated the speed, and hence the average kinetic energy, of its particles increases. If the container is rigid, there is an increased frequency of particle collisions with the sides of the container that, together with the increased momentum of collision, results in an increase in pressure. A graph may be plotted to show how the pressure exerted by a given fixed mass of gas varies as the temperature is changed. The temperature at which this pressure would, in theory, become zero can be determined by extrapolating the curve of this P/T graph to zero pressure. In this experiment you will use a steel bulb connected to a pressure gauge (shown in the figure below). By immersing the bulb in water at various temperatures and reading off the corresponding pressures, a P/T graph can be plotted. This graph can then be extrapolated to determine the zero of absolute pressure, and hence the absolute zero of temperature, which should occur (within experimental error) at approximately 0 K, or ‐273 °C. Furthermore, for a given mass of gas, the pressure divided by the temperature (in K) should be a constant. The apparatus is calibrated to read absolute pressure in lb in–2. In your graph, however, you will be plotting pressure in Pascals (Pa) against temperature in Kelvin (K). To convert lb in‐2 to mmHg (Torr) multiply by 51.70 To convert mmHg to Pascals multiply by 133.3 To convert °C to K add 273.2 The apparatus to be used in Experiment 10a. Experiment 10a 68
EXPERIMENTAL Submerge the bulb successively in: a) boiling water (~100 °C) b) geyser water (~50‐60 °C) c) ice water (~4 °C) recording the temperature and pressure (each to one d.p.). Call a demonstrator to help you change the pressure, and repeat the procedure twice more, so that you have three sets of data of three pressure/temperature readings each. Convert the pressure readings to Pa, the temperature readings to K, and plot P against T for each set of data on the graph paper provided (use the same piece of graph paper for all three graphs). Within experimental error, a straight line should pass through each set of three points and, on extrapolation to zero pressure, indicate that zero pressure and the absolute zero of temperature occur at approximately ‐273 OC, or 0 K. If one (or more) of your data sets does not yield a straight line, then obtain a fourth (or even fifth) set of pressure/temperature readings until you have three sets that are acceptable; consult your demonstrator if you are uncertain. Record the extrapolated absolute zero of temperature, to the nearest 1 K, from each of your three sets of acceptable data. Show that P/T is as constant for your experimental values. 69
Experiment 10a Experiment 10a: The absolute zero of temperature PRE‐LABORATORY EXERCISE 1. Given that 1 atm = 14.70 lb in‐2, show that the conversion factors for: a) lb in‐2 to mmHg (Torr), and b) mmHg to Pascals (Pa) are 51.70 and 133.3, respectively, and determine the conversion factor from lb in‐2 directly into Pa. (1.000 lb in‐2 = 6.892 x 103 Pa) 2. In an experiment to determine the absolute zero of temperature, a student obtained the following pressure/temperature readings. a) Complete the table: P P/T P/T P P T T P/T P/T ‐2
5 ‐2
‐1
‐1
5
‐1
/lb in /mmHg /10 Pa /°C /K /lb in K /mmHg K /10 Pa K /105 Pa °C‐1
20.5 94.6 17.9 55.4 15.4 4.0 Experiment 10a 70
b) c) Under what conditions is P/T a constant? Under what conditions is P/T not a constant? Plot the P/V data on the graph paper provided, extrapolate to the zero of absolute pressure, and read off an estimated value, to the nearest 1 K, for the absolute zero of temperature. __________K
Experiment 10a Experiment 10a: 72
The absolute zero of temperature Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS P /lb in ‐2 1 2 3 4 5 T /°C P /105 Pa T /K P/T /105 Pa K ‐1 P/T /105 Pa °C ‐1 Experiment 10a 73
Extrapolated Values of the Absolute Zero of Temperature: 1. ________ K 2. ________ K 3. ________ K Explain how, by using the apparatus and your graph, you could measure temperature without a thermometer. Experiment 10b Experiment 10b: 75
Calorimetry: Determination of the heat of neutralisation AIM To determine the heat capacity of the calorimeter and then measure the amount of heat generated in a neutralisation reaction. INTRODUCTION Thermochemistry is one branch of the larger division of chemistry known as chemical thermodynamics. It is directly concerned with heats of reaction and the amounts of energy involved during chemical changes. The evolution of heat which accompanies large numbers of chemical reactions ‐ including combustion reactions ‐ is of great importance in technical processes. The heat of reaction is defined as the quantity of heat absorbed at constant volume or constant pressure when the reactants (substances on the left‐hand‐side of the reaction equation) react completely to form the products (substances on the right‐hand‐side of the reaction equation) according to the chemical equation, and the products are brought to the same temperature as the reactants. Reactions in which heat is evolved are called exothermic reactions; those in which heat is absorbed are called endothermic reactions. The object of this experiment is to measure the heat of neutralisation of a strong base by a strong acid. A strong acid or base is a substance which ionises completely or almost completely in solution. A strong acid ionises to give hydronium ions and some anion in solution. A strong base ionises to give hydroxide ions and some cation in solution. When a strong acid reacts with a strong base, the hydronium ions from the acid react with the hydroxide ions from the base to form water. The anion from the acid and the cation from the base generally play no part in the reaction at all and are called spectator ions. e.g. Na+ + OHˉ + H+ + Clˉ → H2O + Na+ + Clˉ When a solution of a strong acid reacts with a solution of a strong base, the heat of neutralisation is therefore the heat of reaction of hydronium and hydroxide ions in the solution to form water: H+ + OHˉ → H2O + heat. It follows that this heat of reaction (expressed per mole of H+ and OHˉ ions reacting) will be the same for any pair of strong acid and strong base, since the reaction is always Experiment 10b 76
between the same species, i.e. hydronium and hydroxide ions. The heat of neutralisation per mole of hydronium and hydroxide ion is equal to about 57.33 kJ. Heats of reaction are usually expressed in the units kJ mol‐1. Heats of reaction are experimentally determined by allowing the reaction to take place in an insulated apparatus called a calorimeter. In practice the heat involved is often determined by noting the change in the temperature of the solution in which the reaction is taking place. The heat capacity of water is 4.18 J K‐1 g‐1. The heat capacity of the solution can be assumed to be the same as the heat capacity of water. Hence the mass of solution times the heat capacity times the change in temperature gives the heat involved. A suitable correction for the heat absorbed by the calorimeter is made in the calculations. The calorimeter, which (in this experiment) consists of the container and a thermometer, obviously undergoes the same temperature change as the solution it contains. This means that some of the heat generated in the reaction is consumed in heating the calorimeter. The extent of this loss must be determined in a separate experiment in order to apply a correction where needed. EXPERIMENTAL The apparatus to be used in this experiment is shown in the figure below. The apparatus to be used in Experiment 10b. Calibration: Determination of the heat capacity of the calorimeter, Ccalorimeter Fill a 250 cm3 beaker with deionised water and heat it on a Bunsen flame to approximately 40 °C. Measure 100 cm3 of deionised water in a measuring cylinder and transfer to the calorimeter. Place the calorimeter on the magnetic stirrer and set to slow stirring speed. Experiment 10b 77
Insert the thermometer, read and record the temperature. Remove the thermometer and place the lid in position. Measure 100 cm3 of the water warmed above into a measuring cylinder. Insert the thermometer, read and record the temperature. Remove the thermometer. Immediately after reading the temperature, lift the plastic lid, pour the warm water from the measuring cylinder into the calorimeter, replace the lid and start the timer. Insert the thermometer through the hole in the lid and secure it in the clamp. Make sure it does not interfere with the rotation of the stirrer bar. Read and record the temperature of the water every minute for five minutes. Record the measurements in the column marked ʺRun 1ʺ on the report sheet. Repeat the above procedure and record the measurements in the column marked ʺRun 2ʺ. Calculations On your graph paper, plot the temperature against the time for both runs. Draw the best straight line through the plotted points and extrapolate back to zero time, thus finding the highest temperature immediately after mixing. Assuming that the density of water is 1.00 g cm‐3 and the specific heat of water is 4.18 J K‐1 g‐1, calculate: (a) the heat lost by the warmer water (in kJ) (4.18 x mass x temp decrease) (b) the heat gained by the cooler water (in kJ) (4.18 x mass x temp increase) The difference between the heat lost by the warm water and the heat gained by the cool water is the heat gained by the calorimeter including the thermometer. If this amount of heat is divided by the increase in temperature experienced by the calorimeter, the heat capacity of the calorimeter can be determined. The calorimeter used in this experiment has a very low heat capacity. The temperature measurements are subject to error, one result of which may be a spurious negative value for the calculated heat capacity. If the average value of the heat capacity of the calorimeter (obtained from the duplicate determinations) turns out to be negative, it is suggested that, for further calculations, the heat capacity of the calorimeter be taken as zero. Determination of the heat of neutralisation Rinse the 100 cm3 measuring cylinder with 10 cm3 of the base solution. Measure 100 cm3 of this solution and transfer to the calorimeter. Insert the stirrer bar and set the stirrer to a slow stirring speed. Insert the thermometer, read and record the temperature. Remove the thermometer and place the lid in position. Experiment 10b 78
Rinse the 100 cm3 measuring cylinder with 10 cm3 of the acid solution. Measure 100 cm3 of this solution in the measuring cylinder. Insert the thermometer, read and record the temperature. Remove the thermometer. Immediately after reading the temperature, lift the plastic lid, pour the acid solution into the base solution in the calorimeter, replace the lid and start the timer. Insert the thermometer through the hole in the lid and secure it in the clamp. Make sure it does not interfere with the rotation of the stirrer bar. Read and record the temperature of the mixture every minute for five minutes. Calculations Plot the temperature against time and extrapolate to zero time so as to obtain the highest temperature reached when mixed. Hence calculate the heat of neutralisation per mole of water produced as indicated on the report sheet. In your calculations you may assume that for all solutions the density is equal to 1 g cm‐3 and the specific heat is 4.18 J K‐1 g‐1. Experiment 10b Experiment 10b: 79
Calorimetry: Determination of the heat of neutralisation PRE‐LABORATORY EXERCISE In an attempt to determine the heat of neutralisation, a student obtains the following results shown below. Caculate Ccalorimeter. Calorimeter calibration Mass of warm water/g 100 Mass of cold water/g 100 Run 1
19.6 Run 2
19.6 Run 3 19.7 Run 1 19.6 51.0 52.6 52.1 51.0 35.3 33.0 36.6 33.3 33.0 Warm water temperature decrease/K 18.0 Cold water temperature increase/K 13.4 Cold water initial temperature/°C Warm water initial temperature/°C Theoretical mixture average temperature/°C Extrapolated mixture final temperature/°C Heat lost by warm water/kJ 7.52 Heat gained by cold water/kJ 5.60 Heat gained by calorimeter/kJ 1.92 Calorimeter temperature change/K 13.4 Ccalorimeter/kJ K‐1 0.143* Average Ccalorimeter/kJ K‐1 Experiment 10b 80
Determination of the heat of neutralisation HCl(aq): 3
Volume/cm 100 Molarity/M 2.556 Initial temperature/°C 21.5 Average initial temperature/°C NaOH(aq): Volume/cm3 Molarity/M Initial temperature/°C 100 2.635 21.3 Run1 Run 2 Run 1 21.4 Extrapolated final mixture temperature/°C 37.0 35.9 37.0 Mixture temperature increase/K 15.6 Heat absorbed by mixture/kJ 13.0 Calorimeter temperature increase/K 15.6 Heat absorbed by calorimeter/kJ 2.23* Total heat absorbed/kJ 15.2 nH2O formed/mol 0.2556 Molar heat of neutralisation/kJ mol‐1 59.5 Average molar heat of neutralisation/kJ mol‐1 *Ccalorimeter 0.143kJ K‐1 Experiment 10b Experiment 10b: 81
Calorimetry: Determination of the heat of neutralisation Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Calorimeter calibration Temperature of cold water at start __________°C (Run 1) _________°C (Run 2) Temperature of warm water at start __________°C (Run 1) _________°C (Run 2) Run 1 Run 2 Time/minutes Temperature Time/minutes Temperature after mixing/EC after mixing/EC Experiment 10b 82
Run 2 Temperature/ °C Temperature/ °C Run 1 time/minutes Heat of neutralisation Temperature of HCl at start _________°C Temperature of NaOH at start _________°C mean temperature _________°C Time/minutes Temperature after reaction/°C Temperature/°C
time/minutes time/minutes Experiment 10b 83
Calculation of the heat capacity of the calorimeter Run 1 Run 2 Mass of water at lower temperature __________g _________g Mass of water at higher temperature __________g _________g Lower temperature _________°C _________°C Higher temperature _________°C _________°C Final temperature immediately after mixing (from graph) _________°C _________°C 1 Heat lost by the warm water _________kJ ________kJ 2 Heat gained by the cold water _________kJ ________kJ Heat lost to calorimeter 1 ‐ 2 _________kJ ________kJ Temperature change experienced by calorimeter __________K _________K Heat capacity of the calorimeter _________kJ K‐1 _________kJ K‐1 Average of values from two runs __________kJ K‐1 Experiment 10b Calculation of the heat of neutralisation of hydrochloric acid and sodium hydroxide Mass of 1 M acid solution __________g Mass of 1 M alkali solution __________g Mean temperature of two solutions __________°C Final temperature immediately after reaction (from graph) __________°C Heat absorbed by aqueous solution* __________kJ Heat absorbed by apparatus (use heat capacity determined above) __________kJ Total heat evolved __________kJ Number of moles of water formed by reaction (use precise molarities given on the blackboard) __________mol Heat of neutralisation (report to 2 significant digits) __________kJ mol‐1 Accepted value (for comparison) __________kJ mol‐1 * Assume a specific heat of 4.18 J K‐1 g‐1 for aqueous solutions. 84
Experiment 11 Experiment 11: 85
pH and indicators AIM To relate pH values to the strength of acids and bases and to become familiar with several indicators. To determine the degree of ionisation of an acid and a base. INTRODUCTION The Brönsted‐Lowry concept of an acid is that an acid is a proton donor, e.g. HF(aq) + H2O(l) → H3O+(aq) + Fˉ(aq). A proton (H+) is transferred from HF(aq) to H2O(l), hence HF(aq) is acting as an acid. The H3O+(aq) molecule is known as the hydronium ion. Rather than express the hydronium ion concentration (acidity) as very small numbers or as exponentials, we use the pH system to express acidity. The pH of a solution is the negative of the base 10 logarithm of the hydronium ion concentration. pH = ‐log10 [H3O+] Similarly, the pOH of a solution is the negative of the base 10 logarithm of the hydroxide concentration. pOH = ‐log10 [OHˉ] At 25 °C, pH + pOH = 14.00. In general solutions that have: pH < 7 are acidic pH = 7 are neutral pH > 7 are basic The adjectives, strong and weak, as applied to acids, bases, or salts, refer to their relative degrees of ionisation. A strong acid is “strong” – that is, very reactive as an acid − because of the high concentration of hydrogen ion (H+) that it contains in solution. A weak acid, although able to neutralise as much base as an equivalent amount of a strong acid, is “weak” because only a small proportion of its molecules are dissociated into hydrogen ion and the anion. It is, therefore, less reactive as an acid. Correspondingly, the strength of a base depends on the extent to which its molecules are dissociated into the cation and hydroxide ion. Experiment 11 86
As discussed previously, an indicator is a complex organic compound the colour of which, when in aqueous solution, depends on the relative concentration of hydrogen and hydroxide ions in the solution. In this experiment, you will prepare a series of solutions of known hydrogen ion and hydroxide ion concentration. This may be done by making dilutions of 0.1 M solutions of the strong acid hydrochloric acid, and of the strong base sodium hydroxide, respectively. These substances are thought to be completely ionised in dilute solution, so the molarity of the hydrogen ion and of the hydroxide ion, respectively, will be the same as the concentration of the corresponding acid or base solution. This series of solutions will be used to learn the colours of several indicators, and the concentration range through which a change of colour takes place with each indicator. Also, using this method, the approximate degree of ionisation of acetic acid and of ammonium hydroxide will be determined, each at two different concentrations. It should be noted that the hydrogen ion or hydroxide ion concentration in a solution can be estimated closely by one indicator only if the concentration lies within the colour change range of that indicator. EXPERIMENTAL Preparation of solutions of known H+ concentration Prepare solutions of 0.01 M H+, and 0.001 M H+, by dilution of 0.1 M HCl, as follows. First, thoroughly clean two 100 or 250 cm3 beakers or flasks. Rinse these with tap water, then, with not more than 5 ml of distilled water from the wash bottle. By means of a measuring cylinder, carefully measure 5.0 cm3 of 0.1 M HCl. Add distilled water to give a total volume of 50.0 ml. Pour this back and forth between the measuring cylinder and one of the clean flasks several times to mix it thoroughly. Keep this solution in a labelled flask. Leave 5.0 ml (carefully measured) of the solution prepared above in the measuring cylinder and make a second tenfold dilution by filling carefully to the 50.0 cm3 mark with distilled water. Mix as before and transfer to a second labelled flask. More dilute solutions, from 10−4 M to 10−7 M H+, are already prepared for your use. These solutions of very low H+ concentration are “buffered” solutions. That is, there are substances present in the solution that keep the H+ concentration in the solution very nearly constant, even when some additional acid or base is added. Without these buffers the carbon dioxide from the air reacts with the water, and forms too much H+ to maintain a known concentration. The colours of indicators in acid solutions Prepare a series of clean test tubes containing 5 cm3 each of the acid solutions ranging from 10−1 to 10−7 M H+. Add to each test tube one drop of methyl violet indicator. Stir each with a glass rod and note the colours which are characteristic of the given H+ Experiment 11 87
concentrations. The methyl violet in the stronger acid solutions will fade on long standing. Compare indicator colours by looking lengthwise through the tubes against a white background (see figure below). Save the tubes covering the colour change range (three or four tubes) and label each. Now prepare another series of tubes containing 5 cm3 each of the acid solutions from 10−1 M to 10−7 M H+, and test each with one drop of methyl orange indicator. Stir each and note the colours which are characteristic of the given H+ concentrations. Again save the tubes covering the colour change, labelling each. The H+ concentration of an unknown solution Take a clean test tube, labelled with your name, to your demonstrator and obtain a solution of unknown H+. Test portions of this solution with methyl violet and methyl orange. Record the H+ concentration of your unknown solution on your report sheet. The degree of ionisation of acetic acid Obtain a 5 cm3 portion of 1 M HC2H3O2, and test it with one drop of methyl violet indicator. Make as accurate an estimate of the H+ concentration in the solution as possible, by comparison with your standards. If the colour lies between the colours of two of your standards, try to estimate the H+ concentration in between the values represented by these standards. Calculate the concentration of acetate ions in this solution, the concentration of unionised acetic acid molecules, and the fraction of all the acetic acid molecules in solution that are ionised (express as percent ionisation). Also test a 5 cm3 sample of 0.1 M HC2H3O2 with both methyl violet and methyl orange to estimate the H+ concentration. Calculate the C2H3O2− concentration and the concentration of unionised HC2H3O2. Calculate the percent ionised acetic acid and compare with the percent ionised for a 1 M solution. The preparation of solutions of known OH− concentration Prepare a 50 cm3 sample of 0.01 M NaOH from the stock 0.1 M NaOH solution by tenfold dilution as you did for HCl. Be careful to rinse all vessels used. Keep the solutions stoppered in order to prevent the absorption of carbon dioxide from the air. As with the Experiment 11 88
acid solutions, the more dilute basic solutions, from 10−3 to 10−7 M OH−, are already prepared for your use. They are also buffered solutions. The absorption of carbon dioxide from the air into an unbuffered basic solution would be even more rapid and troublesome than in the case of dilute acid solutions. The colours of indicators in basic solutions This time you will use three different indicators, namely, indigo carmine, alizarin yellow R, and phenolphthalein, to identify the hydroxide ion concentration of solutions. Prepare a series of test tubes containing 5 cm3 each of the alkaline solutions ranging from 10−1 to 10−7 M OH−. Test each with two drops of indigo carmine indicator. (Indigo carmine indicator does not keep very well. If it is not a deep blue colour ask your demonstrator to obtain some freshly prepared indicator solution.) Stir each test tube and note the colours and, in particular, the range of the colour change. Keep three or four tubes which cover the range of the colour change and label each for use later. Likewise, determine the colours and colour range with alizarin yellow R and with phenolphthalein. Keep the tubes covering the colour change in each case for later use. The degree of ionisation of ammonia in solution Ammonia in solution ionises to form NH4+ and OH‐ ions. Test 5 cm3 portions of 1 M and of 0.1 M ammonia solutions with indicators to determine the hydroxide ion concentration in each case. Calculate the concentration of NH4+ and of unionised NH3 in each case. Reactions involving weak acids and weak bases Add a drop of methyl violet indicator to 5 cm3 of 0.1 M HCl in a test tube, and then gradually add 1 to 2 cm3 of 1 M NaC2H3O2 solution. Try the same experiment again except this time use 1 M NaCl in place of the 1 M NaC2H3O2. Also try 1 M NH4C2H3O2 instead of 1 M NaC2H3O2. Explain the results and write the net ionic equation for any reaction taking place. Add a drop of alizarin yellow R to 5 cm3 of 0.1 M NaOH solution in a test tube, and then gradually add 1 to 2 cm3 of 1 M NH4Cl solution. Try the same experiment again, except use 1 M NaCl in place of the 1 M NH4Cl. Also try 1 M NH4C2H3O2 instead of 1 M NH4Cl. Explain the results and write the next ionic equation for any reaction taking place. Experiment 11 Experiment 11: 89
pH and indicators PRE‐LABORATORY EXERCISE 1. Complete the following table. H+ concentration/ M pH
2.31 OH‐ concentration/ M pH
8.5 x 10‐5 6.52 2. A 0.1 M solution of a weak acid has a pH of 5. Calculate the percentage of the acid that is ionised. Experiment 11 Experiment 11: 90
pH and indicators Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS H+ concentration of solution prepared by diluting 5 cm3 of 0.1 M HCl to 50 cm3 H+ concentration of second solution prepared by dilution _____ M _____ M The colours of indicators in acid solutions H+ concentration 10—1 M 10—2 M 10—3 M 10—4 M 10—5 M 10—6 M 10—7 M pH
Methyl violet
Methyl orange
Experiment 11 91
Unknown acid solution colour with methyl violet ________________ colour with methyl orange ________________ H+ concentration ________________ pH of unknown solution ________________ The degree of ionisation of acetic acid Complete the table. Show all calculations in the space given below. Solution Methyl Methyl H+ C2H3O2‐ HC2H3O2 violet orange concentration concentration concentration
1 M HC2H3O2 0.1 M HC2H3O2 % ionised Does the degree of ionisation of acetic acid increase or decrease as the dilution is increased? Experiment 11 92
The colours of indicators in basic solutions pH Phenolphthalein
Alizarin yellow
R 10—3 M 10—4 M 10—5 M 10—6 M 10—7 M OH‐ concentration 10—1 M 10—2 M Indigo carmine The degree of ionisation of ammonia in solution Complete the table. Show all calculations in the space given below. Solution Indigo Alizarin Phenol‐
OH‐ concn NH4+ concn carmine yellow R phthalein 1 M NH3 0.1 M NH3 NH3 concn % ionised Experiment 11 93
Reactions involving weak acids and weak bases Solutions mixed
HCl + NaC2H3O2 Indicator used and colour change HCl + NaCl HCl + NH4C2H3O2 NaOH + NH4Cl NaOH + NaCl NaOH + NH4C2H3O2 Explain any differences in the effects of the salts on HCl, and give the net ionic equation for any reaction occurring. Explain any differences in the effects of the salts on NaOH, and give the net ionic equation for any reaction occurring. 94
Experiment 12 Experiment 12: Chemical reactions Kinetics: Rates of chemical AIM To study the rate of the reaction between thiosulfate and hydronium ions, and to determine how a change in thiosulfate ion concentration affects the rate of the reaction. INTRODUCTION Chemical kinetics is the study of the rates of chemical reactions and the mechanisms by which they occur. The rate of a reaction describes how rapidly chemical change occurs. The mechanism describes the series of steps or the pathway by which reactants form products. Some reactions, such as the neutralisation of an acid by a base or the combustion of petrol in oxygen, are very rapid. Other reactions, such as the rusting of iron, can be very slow. In this experiment, you will study a reaction that has a moderate rate that can be measured several times in one laboratory period. Consider the hypothetical reaction R → P As the reaction proceeds, the reactant concentration [R] decreases and the product concentration [P] increases. The rate of the reaction is thus the change in reactant concentration with time, ‐ Δ[R]/Δt or the change in product concentration with time, +Δ[P]/Δt. In practice initial rates (the rate at the beginning of the reaction) are usually measured because the concentrations of all reactants are known at the start of a reaction. The rate of a specific chemical reaction generally depends upon the following factors: •
chemical nature of the reacting species •
concentrations or partial pressures of the reacting species •
surface areas of the reacting species (in heterogeneous reactions involving solids) •
reaction temperature •
presence or absence of a suitable catalyst. To study the influence of one factor on the rate of a reaction, the other factors must be kept constant. By studying a single chemical reaction in this experiment, you will be keeping the first factor constant. Experiment 12 95
For a reaction to occur, the kinetic theory proposes that •
reacting molecules must come into contact (collide) with one another •
the colliding molecules must do so with enough energy (the energy of activation, Ea) for the collision to result in a reaction occurring •
the molecules must have the right orientation for an effective collision. An increase in the number of reactant molecules in a given volume increases the reaction rate through all three of these factors ‐ the number of collisions increases, the number of molecules with the required energy (Ea) increases, and the number of molecules with the correct orientation increases. An increase in temperature increases only the number of molecules with the required energy, which is why reactions go faster when they are heated. Catalysts may interact with reactants to lower the amount of energy required to cause a reaction to occur, which has the same effect: a larger fraction of the reactants will have this energy and so the rate increases. When surface area in a heterogeneous reaction is increased, the frequency of collisions increases and so the rate of reaction also increases. The relationship between the rate of a chemical reaction and the concentration of the reactants is called a rate law. Consider the reaction aA + bB → cD + dD. The rate law expression for this forward reaction is rate = k[A]x[B]y. The powers x and y to which concentrations of the reactants A and B are raised are not necessarily the same as the stoichiometric coefficients a and b for these reactants, and must be determined by experiment. These exponents may be zero, small whole numbers, or even fractions. The constant k in the rate‐law equation is called the rate constant. Its value depends upon all the factors that affect the rate of reactions given above, except concentration. The sum of the exponents of the concentrations in the rate‐low expression is called the order of the reaction. For the reaction S2O82‐(aq) + 2I‐(aq) → 2SO42‐(aq) + I2(aq) experiments have shown that if [I‐(aq)] is held constant while [S2O82‐(aq)] is doubled the rate of the reaction increases by a factor of 2. Therefore, the rate is first order in S2O82‐(aq), or rate = k’[S2O82‐(aq)] where k’ = k[I‐(aq)]y is constant. Additional experiments in which [S2O82‐(aq)] was held constant while [I‐(aq)] was doubled were also found to double the rate of reaction. Therefore, the rate is first order in I‐(aq), or rate = k’’[I‐(aq)] where k’’ = k[S2O82‐(aq)]x is constant. Experiment 12 96
The overall rate law for the reaction is: rate = k[S2O82‐(aq)][I‐(aq)]. and the overall reaction is second order. The reaction to be studied in this experiment is the acidification of sodium thiosulfate (Na2S2O3) to give sulfur dioxide gas and a precipitate of sulfur: S2O32‐(aq) + 2H3O+(aq) → SO2(g) + S(s) + 3H2O(l). This reaction occurs in several stoichiometric steps: S2O32‐(aq) + 2H3O+(aq) → H2S2O3(aq) + 2H2O(l) H2S2O3(aq) → H2SO3(aq) + S(s) H2SO3(aq) → SO2(g) + H2O(l). The mechanism of this reaction is too complex for complete study. For simplification [H3O+(aq)] will be kept constant while [S2O32‐(aq)] is varied, and the time taken for one of the products (in this case a precipitate of sulfur) to appear will be recorded. This is a measure of the rate of the reaction: the more rapidly the reaction is taking place, the smaller will be the time required to build up a visible precipitate of sulfur. The reaction rate is thus inversely proportional to the time taken for the precipitate to appear: reaction rate ∝ [S2O32‐(aq)] ∝ 1/time or reaction rate = ‐Δ[S2O32‐(aq)]/Δt ∝ 1/time A plot of initial [S2O32‐(aq)] against 1/t shows how the rate responds to a change in [S2O32‐
(aq)], from which a rate law expression of the form rate = k’[S2O32‐(aq)]x (where k’ = k[H3O+(aq)]y is constant) can be derived. Experiment 12 97
EXPERIMENTAL Dilute 1.0 M Na2S2O3 solution as necessary to obtain 10 cm3 each of 0.80 M, 0.60 M. 0.40 M and 0.20 M Na2S2O3 solution. For each solution in turn, i) Measure out 5 cm3 into the large test tube. Add 5 cm3 0.5 M HNO3(aq), starting the stop‐watch at the moment of addition. Shake well to mix the solutions. ii) Note the time taken for the solution to go cloudy as the precipitate of sulfur appears. (It does not matter how cloudy the solution goes before the time is measured, as long as all solutions are timed to that same degree of cloudiness. An easy way to measure ‘cloudiness’ is to make an ‘X’ on a piece of white paper and place this behind the solution. The solution is cloudy when the ‘X’ can no longer be seen.) Plot a graph of [S2O32‐(aq)] against t, and a second graph of rate (1/t) against [S2O32‐(aq)]. Comment on the shapes of the graphs, and derive the mathematical expression relating concentration to the rate of the reaction. 98
Experiment 12 Experiment 12: Chemical reactions Kinetics: Rates of chemical PRE‐LABORATORY EXERCISE Rate data for the overall reaction 2NO(g) + O2(g) → 2NO2(g) are tabulated below. Experiment [NO]i/M
[O2]i/M
1 2 3 0.0125 0.0250 0.0125 0.0126 0.0252 0.0252 Initial Rate/10‐2 M s‐1 1.41 11.3 5.64 Determine the rate law for this reaction, the order in each reactant, and the overall reaction order. (rate = k[NO(g)]2[O2(g)]; 2nd order in NO(g); 1st in O2(g), 3rd order overall) 99
Experiment 12 Experiment 12: Chemical Kinetics: Rates of Chemical Reactions Pre‐Lab/ Prac/ Deductions (sig. fig. etc.) Total/ Mark Name: _____________________________
Student number: __________________________
Date: _____________________________
Lab. number: _____________________________
Seat number: _____________________________
Demonstrator: ____________________________
RESULTS Solution 1.0 M Na2S2O3(aq)
volume used/cm3 Rate/s‐1 1.0 M 0.80 M 0.60 M 0.40 M 0.20 M Plot of [Na2S2O3(aq)] vs t: Rate law expression: time/s
Plot of rate vs [Na2S2O3(aq)]: Appendix 1 Appendix 1: 100 Laboratory apparatus Each student workbench is supplied with the following apparatus, which should be returned to its correct locker at the end of each laboratory session. Description Quantity Item 3
Erlenmeyer (conical) flasks 250 cm 4 3
Erlenmeyer (conical) flasks 50 cm 1 3
Beakers 100 cm 2 3
Beakers 50 cm 2 3
Measuring cylinder 50 cm 1 3
Measuring cylinder 10 cm 1 Volumetric flask 250 cm3 1 Pyrex filter funnel 45 mm diameter 1 Pyrex filter funnel 75 mm diameter 1 Weighing bottles No. 4 vial 2 Weighing boat (plastic) 1 Pipette filler Pi‐pump type 1 Glass rod 4mm diameter. 1 Droppers and teats Pasteur 2 Buchner funnel 5.5 cm 1 Hirsch funnel 45 mm 1 Pasteur pipette 1 Pyrex filter flask 100ml 1 Spatula (medium) 1 Retort Clamps 1 Retort ring 1 Boss heads 1 Washbottle Plastic‐250 cm3 1 Wooden peg 1 Test tube rack and test tubes 1 Test tube brush (small) 1 Gauze mats 1 Nutec Mat 1 Mini Quickfit clamp 1 Rubber adaptor for Buchner funnel 1 Ice bucket 1 Vacuum pipe 1 Bunsen burner (on bench) 1 Appendix 1 101 Appendix 1 102 Appendix 2 Appendix 2: 103
The laboratory balance Of all the instruments used by a chemist, the balance is the most important. It is, therefore, essential that students should learn to weigh accurately and rapidly from the beginning. However, a balance is an extremely delicate instrument and in order that it should retain its accuracy it is imperative that it is handled with the utmost care. The most important attributes of a chemical balance are its sensitivity and rapidity of action, and the student should appreciate that, since most errors in analysis arise from faulty weighing, it is impossible to take too much care using the balance. Using the Balance The balances used in this laboratory are both extremely expensive and highly sensitive! Please be CAREFUL when using them! 1. Do not move the balance on the balance bench. 2. Never touch the balance pan with your fingers, or breathe on it. 3. Items to be weighed must always be at room temperature. 4. Never weigh corrosive or volatile substances in an open vessel. A stoppered weighing bottle should be used for such substances to eliminate the danger of corrosion. Do not spill chemicals on the balance pan, in the balance or on the balance bench. To minimise this danger, when weighing a substance, never add or remove any of the substance from the weighing receptacle while it is on the balance pan. First remove it from the balance case and then add or remove substance as necessary. 5. Report immediately any accidental spilling of chemicals on the balance to your demonstrator so that the spill may be cleaned at once. 6. Please leave the balance in the condition in which you would wish to find it, i.e. perfectly clean. Appendix 3 Appendix 3: 104
Volumetric apparatus ERROR OF PARALLAX Volumetric appararus should be read with your eye on the same level as the meniscus (curved surface of the liquid in the vessel). If this is not done, an incorrect reading will be taken. This source of error is known as an error of parallax. Always read the bottom of the meniscus. THE PIPETTE The laboratory pipette is a glass vessel which delivers a definite volume of liquid under certain specific conditions. It consists of a cylindrical bulb with tubes at each end. Pipettes come in different sizes, ranging from 1 to 250 cm3. The letter B on the bulb signifies that the pipette is a “B” grade pipette, i.e. the manufacturer guarantees that the volume delivered will be between ± 0.05 cm3 of the stated volume. “A” grade pipettes are guaranteed to deliver between ± 0.01 cm3. The pipette Appendix 3 105
Washing and preparing the pipette The pipette should always be assumed to be dirty, and must be rinsed with both tap water and de‐ionised water, and then three times with the solution to be dispensed. After rinsing the pipette with both tap water and de‐ionised water, dispense ~20 cm3 of the solution provided into a clean, dry 100 cm3 beaker. Insert the upper end of the pipette into the lower end of the pipette filler and rotate the pipette carefully to work no more than 0.5 cm of the glass bore into the rubber sleeve (If you push the pipette further in the filler will not work properly). Do NOT suck up solutions by mouth; use the pipette filler at all times for filling the pipette with solution. Use the wheel on the pipette filler to suck solution into the pipette. Remove the pipette from the solution, detach the filler and immediately place your finger over the end (why?). Hold the pipette as level as possible, and rotate the pipette on the fingertips to ensure that solution flushes over the entire inner surface, including a length of 2‐3 cm above the graduation mark. Allow the pipette to drain through the lower end only, and repeat the process twice. Using the Pipette In volumetric analysis the procedure of pipetting is the single largest source of error for beginners. The technique of pipetting requires careful practice, and will be demonstrated to you, in addition to the notes on the pages that follow. If a thorough study is made of these pages before the laboratory session, you should be able to follow the demonstration and subsequently be able to use the pipette correctly and accurately. After preparing the pipette, discard the remainder of the solution in the 100 cm3 beaker and refill it. Reassemble the pipette and pipette filler and draw up solution sufficient to fill the pipette to ~2‐3 cm above the graduation mark on the pipette (a). Do not allow any Appendix 3 106
liquid to enter the pipette filler; if you accidentally do so, or you think that the filler has liquid in it already, show your demonstrator. Remove the pipette filler and place your finger over the open end of the pipette (b). Wipe the outside of the pipette with a tissue, being careful not to touch the point of the pipette with the tissue otherwise solution will be lost by capillary action (c). Keeping the pipette at eye level and in a vertical position (using both hands), allow the liquid level to fall by lifting your finger slightly, so that the bottom of the meniscus becomes just level with the graduation mark on the pipette. If a small drop of solution remains at the pipette tip at this stage, it can be removed by touching the bottom of the pipette against the side of the beaker (d). Replace the beaker under the pipette with a conical flask, and, again keeping the pipette vertical, remove your finger to enable the solution to drain into the flask. Once the solution has finished draining, touch the bottom of the pipette against the side of the conical flask. The liquid remaining in the tip of the pipette at this stage must not be blown out into the conical flask, as this extra drop is taken into account when the pipette is calibrated. Once pipetting is complete, clamp the pipette on the burette stand to prevent accidental breakage. Do not leave the pipette on the bench top where it can become contaminated. THE BURETTE A burette consists of a tube of uniform bore graduated in cm3 and tenths of a cm3. The volume delivered can be read on the graduations accurately to the first decimal, i.e. to 0.1 cm3. Readings are recorded to the nearest 0.02 cm3 by estimation. The burette Appendix 3 107
Washing and preparing the burette A burette should always be assumed to be dirty, and must therefore be rinsed thoroughly before use, both with tap water and then with de‐ionised water. It is then “prepared” by rinsing the inside with the solution to be used. After rinsing the burette with both tap water and de‐ionised water, dispense ~25 cm3 of the solution provided into a clean, dry 100 cm3 beaker. Pour about ~8 cm3 of this solution into the burette (make sure that the burette tap is closed if you don’t want to go home with wet feet!), hold the burette as level as possible, and rotate on the fingertips to ensure that solution flushes over the entire inner surface. Drain through the stopcock to make sure that the solution runs freely and is not obstructed. Repeat twice, draining the final rinse through the open end of the burette. Discard the remainder of the solution in the 100 cm3 beaker and refill it, then use the beaker to fill the burette to above the zero mark. Open the stopcock to displace air from the jet and to ensure that the jet is completely filled with solution. You will be shown how to remove persistent air bubbles. Finally allow to drain/top up until the meniscus lies between 0‐1 cm3. Place the burette in the burette stand with a tissue under the stopcock while preparing the remainder of the glassware for the titration; if the stopcock leaks, even very slowly, the wet tissue will alert you and you can remedy the problem before wasting time performing inaccurate and meaningless titrations. Using the Burette After pipetting, the greatest sources of error in volumetric analysis stem from students not being able to master the techniques of estimating the volume correctly, and adding a single drop from a burette. Adding a single drop of solution is a technique that requires a little practice. For a right‐handed person it is customary to swirl the conical flask with the right hand whilst operating the tap with the left hand, as shown in the picture. You will be shown how to do this ‐ ask your demonstrator to check that you are doing it correctly. Appendix 3 108
As mentioned before, only the first decimal place of a volume can be read directly from the burette; the second decimal place is then estimated to the nearest 0.02 cm3. Being able to estimate correctly is thus of great importance if your volumetric analyses are to be accurate. Study the sketch below to give you an idea, and then get your demonstrator to check that you are doing it correctly. 21
21
21
21
21
22
22
22
22
22
21.30
21.34
21.36
21.38
21.40 Placing a white card below the level of the meniscus, as shown in the diagram alongside, also aids in taking an accurate reading from a burette. The pipette and burette should be clamped safely when not is use, as shown in this figure. Appendix 4 Appendix 4: 109
Experimental errors INTRODUCTION In nearly all scientific endeavours, measurements are made and the data so obtained used in calculations to arrive at a result on which a final conclusion is based. In practice it is seldom possible to make exact measurements. There are unavoidable errors inherent in the apparatus used, in the methods employed and in the observational powers of the experimenter. In some instances, errors may not affect the result or the conclusion, but in most instances the result is open to a degree of doubt and the extent of the error in the result must be conveyed by following established convention. An example of the first instance is the result and conclusion based on the following data: The mass of a consignment of apples is 123 kg. If the mass of a box of apples is 20 kg, how many boxes are there in the consignment? The result of dividing 123 kg by 20 kg per box is 6.15 boxes, but the conclusion will be that there are 6 boxes in the consignment. An example of the second instance is a determination of the STP molar volume of a gas which gave a value of 22.1 dm3. As the accepted value is 22.4 dm3, it is obvious that errors inherent in the apparatus, method and operation have (in combination) caused a deviation of 0.3 dm3 from the accepted value, i.e. an error of 0.3 × 100
= 1.3% . 22.4
SOURCES OF ERROR In scientific endeavour, errors arise from: •
imperfections in apparatus •
imperfections in method •
variations in the environment •
imperfections in technique. Imperfections in apparatus Most of the apparatus used in a first course are produced in large batches in order to reduce cost. Consequently the items are not individually calibrated. However, the manufacturers issue certificates with each batch indicating the limit of inherent error in any one item of a batch. For example: a B grade burette certificate may state: Tolerance 50 cm3 ± 0.1 cm3 and a B grade pipette certificate may state: Tolerance 10 cm3 ± 0.05 cm3. These statements mean that a titration volume could be in error up to a maximum of 0.2% Appendix 4 110
when using a B grade burette and a 10 cm3 aliquot pipetted by means of a B grade pipette could be in error up to a maximum of 0.5% due to imperfections in the shapes of these glass vessels. Mass measurement apparatus has much lower tolerances than volume measurement apparatus, and, for all practical purposes, mass measurement errors are negligible in comparison with volume measurement errors. Imperfections in method Imperfections in method likely to be encountered in a first course are: solubility of a precipitate, co‐precipitation, decomposition and/or volatilisation during drying in an oven, absorption of moisture or carbon dioxide from the air, oxidation on exposure to the atmosphere, attack on glass vessels by caustic substances, etc. Variations in the environment Variations in temperature, pressure and humidity of the air in a laboratory occur due to change in weather conditions, change of the seasons and diurnal fluctuations. Imperfections in technique Operator error is usually far greater than all previously mentioned errors combined. Some errors are unavoidable, for example the perception of indicator colour change is a subjective judgement on the part of the operator and is bound to differ from the perceptions of other operators. Avoidable errors are many and often occur because of inattention. The most frequent and serious error in a first course is incorrect pipetting procedure. Next come the errors due to ineffectual washing of a precipitate and excessive washing of a precipitate. More serious is physical loss of reagent through spillage. In assessing the results of first year students in quantitative analysis, an error of 2% is regarded as acceptable and may earn full marks. This 2% is made up of 1% operator error and 1% for all other sources of error combined. SIGNIFICANT DIGITS If, in the example above of the determination of STP molar volume, the experimenter reported his result as 22.100 dm3, he would have been guilty of an absurdity. The accepted value of 22.414 dm3 has been obtained in advanced research using sophisticated equipment and working under ideal conditions. According to the conventions of statistics, the number 22.414 guarantees the value to be closer to 22.414 than it is to 22.413 and to 22.415, i.e. it lies within the range 22.4135 and 22.4145. (If the uncertainty had been greater, the range would have been given as, e.g. 22.414 ± 0.001). When given as 22.414, the uncertainty, and therefore the likely error, is: 0.001 × 100
= 0.00446% . i.e. less than 0.005%. 22.414
Appendix 4 111
In terms of the 2% error allowed in a first course, a result within the range 22.4 ± 22.4x0.02 (i.e. within the range 22.8 and 22.0) would have been acceptable. Thus even if the experimenter’s calculator showed a final result of say 22.100, the result is still reported as 22.1 in order not to claim a degree of certainty that does not exist. To be able to decide on the correct procedure, the student must understand the meaning of the term significant digits. The number system has ten digits 0, 1, 2… 8, 9. Any given number/figure/value consists of one or more digits. Thus the statement “12 apples” leaves no doubt as to the quantity. The number has been obtained by counting, and, except for a blunder, is definite and indisputable. However, a statement such as “12 km” is the outcome of a measurement and immediately the question arises how the measurement was made. If the person making the statement had measured the distance by walking, the reader would interpret the distance as 12 ± 1 km or even 12 ± 2 km, knowing how fallible the measuring procedure was. If the measurement had been made by a surveyor using a tellurometer, he would give the distance as 12.0000 km. The four zeroes following the decimal are not an attempt at pedantism, they are given deliberately to assure the reader that the surveyor is prepared to guarantee that the value is closer to 12.0000 than it is to 11.9999 or to 12.0001, i.e. it lies within the range 11.9995 and 12.0005. The surveyor thus implies that his error is not greater than 1 dm. In statistical language it is said that the number is given to six significant digits, the first five being beyond dispute and the last digit being uncertain because it is a “best estimate”. Whereas the zeroes in the surveyor’s 12.000 km are deliberate and therefore significant, the zeroes in the distance 0.0012 km are not significant because they can be dispensed with by reporting the distance as 1.210‐3 km or 1.2 m or 12 dm. In all three forms there are two significant digits. However, if this distance were given as 120 cm, it would have three significant digits and thus claim greater accuracy, i.e. a smaller uncertainty. The value 12 dm implies that the distance lies within the range 11.5 and 12.5 dm – a variation of 1 dm or 10 cm. The value of 120 cm implies that the distance lies within the range 119.5 and 120.5 cm – a variation of 1 cm or 0.1 dm. Similarly, if the distance were given as 1200 mm, i.e. to four significant digits, the variation would be 1 mm or 0.1 cm or 0.01 dm. Round numbers such as 100, 10 and 1 are considered to have only one significant digit because the zero’s merely indicate the order of magnitude. Appendix 4 The first rule of significant digits Measurements, i.e. observed quantities, should be recorded with one uncertain digit retained. Figure 1 illustrates this rule. It shows the level of titrant in a burette. It is clear that the volume is more than 27.4 but less than 27.5 cm3. The experimenter has made an estimate of the interval beyond 27.4 and arrived at the fraction ⅔. He has thus recorded the volume as 27.4 + ⅔ × 0.1 = 27.4 + 0.07 = 27.47 cm3 and will use this value in subsequent computations. The value 27.47 consists of four significant digits, the first three indisputable and the last uncertain because it has been estimated. 112
The second rule of significant digits In carrying through a series of computations, one digit beyond the last significant digit must be retained in order that the last significant digit is not altered in the computational process. This rule can be illustrated by the following example: If the experimenter above did two further titrations and obtained say 27.42 and 27.40 cm3 in his second and third titrations, his calculator would show the mean value as 27.433333 cm3. In further computations the experimenter must use the value 27.433 cm3 in order to conform to rule 2. However, if the experimenter obtained say 27.32 and 27.50 cm3 in his second and third titrations, his mean would still be 27.43333 cm3 but now it would be absurd to use 27.433 cm3 in further computations as his third digit is already uncertain. He should now use 27.43 cm3 as the mean value in further computations. The third rule of significant digits In a result, there must be as many digits as will give one and only one uncertain digit. This rule has been re‐phrased in a rather simplistic form to read: the result cannot contain more digits than the factor with the fewest significant digits. This rule can be illustrated by carrying the example used above to completion. The mean of three titrations was 27.433 cm3. If the molarity of the titrant was, say 0.1013, and the aliquot 10 cm3 (measured by means of a pipette) the calculation of the unknown concentration is: 27.433 cm 3 x 0.1013 M
= 0.2778962 M . unknown molarity =
10 cm 3
Appendix 4 113
As the volume of the aliquot, 10 cm3, appears to have only one significant digit, the result must be recorded as 0.2 M according to rule 3. However, the term “10 cm3 pipette” merely indicates an order of magnitude and does not describe the capacity properly. The manufacturer’s certificate gives the tolerance of a 10 cm3 pipette as a 0.05 cm3. The lower value, 9.95, has three significant digits and this fixes the number of significant digits in the result at three. Thus the result is given as 0.278 M. Had the calculator shown a final value of say 0.27741879 M, the value reported would be 0.277 M to conform to the rule of rounding off. The fourth rule of significant digits The precision of a result must not be diminished by a computation. In the calculation 0.081 × 141.8 = 11.4858 the factor 0.081 contains two significant digits and the inclination might be to limit the product to two significant digits, i.e. to report the product as 11 according to the simplistic version of rule 3 above. Although the factor 0.081 has only two significant digits, its uncertainty is 1 in 81 whereas the uncertainty of the product is 1 in 11. Thus the precision of the result has been diminished. In order to conform to rules 3 and 4, the result must be reported as 11.5. The computations below show that the first decimal place is the first variable digit, 0.0805x141.8 = 11.4149 0.0815x141.8 = 11.5567 and a result must always contain one uncertain digit according to rule 3. By contrast, in the calculation 0.012x141.8 = 1.7016 the product would be given to two significant digits, viz. 1.7 because the second digit in 1.7 is uncertain as shown by the computations below: 0.0115x141.8 = 1.6307 0.0125x141.8 = 1.7725 Appendix 4 114
Multiplication or division by a factor merely changes the order of magnitude; it does not affect the precision. Thus if 100.0 g is required to make 1000 cm3 of 1 M solution, then the mass required to make 200 cm3 of 0.1 M solution is: 200 cm 3 0.1 M
100.0 g ×
×
= 2.000 g . 1000 cm 3 1.0 M
Subtraction can reduce the number of significant digits. When the mass of substance is obtained by difference, then the difference of two balance readings will have fewer significant digits than the balance readings. e.g. mass of vial + substance 12.106 g 5 significant digits mass of vial 11.271 g 5 significant digits mass of substance 0.835 g 3 significant digits The converse is not true, i.e. addition cannot increase the number of significant digits, e.g. 0.603 + 0.731 = 1.33 g. DRILL PROBLEMS (You may refer to the answers to some of these problems, after solving them on your own initiative). 1. Carry out the operations on the data given in each of the following cases to calculate the quantity called for. Show your method, including the dimensions of measurement. (These units will tell you which mathematical operation to perform). (a) Velocity = 50 km h‐1, time = 0.5 h, distance = ? (b) Velocity = 300 000 km s‐1, distance = 150 000 000 km, time = ? (c) Time = 9.3 s, distance = 100 m, velocity = ? (d) Density Al = 2.70 g cm‐3, mass = 2700 g, volume = ? (e) Mass Hg = 272 g, volume = 20 cm3, density = ? (f) Mass apples = 120 kg, mass in each box = 20 kg box‐1, number of boxes = ? (g) Mass H2O = 180 g, molar mass = 18 g mol‐1, number of moles = ? 2. How many significant digits are there in each of the following numbers? (a) 3005 (d) 0.350 (b) 3500 (e) 3.050 (c) 0.035 (f) 3.0005 Appendix 4 115
3. Carry out the following operations, recording the answer correctly in accordance with the rules of significant digits: (a) Subtract 5.1 from 28.347 (b) Subtract 5.10 from 28.347 (c) Multiply 0.020 by 1.111 (d) Divide 36.02 by (3.0)2 4. A beaker of water has a mass of 1200 grams. How would you write this number so as to avoid ambiguity, if the mass is known to the nearest: (a) ten grams (b) one hundred grams (c) gram, (d) tenth of a gram. 5. The length of a table is measured as 2 metres, 3 centimetres, and 4 millimetres. Express this length as: (a) metres, (b) centimetres, (c) millimetres (d) kilometres. How many significant digits in each case? 6. A series of beakers have the following masses: 125.2 g, 90.3 g, 56.2 g and 20.237 g. How should you record the sum of these masses so as to avoid any incorrect conclusions as to the precision of measurements? 7 Three determinations of the percentage of chlorine in sodium chloride were 60.1%, 60.5% and 60.3%, averaging 60.3%. The accepted value, based on the atomic masses (Na 22.9979 amu; Cl 35.4571 amu), is 60.650% Cl. What is the percentage error in the analysis, and to how many significant digits should it be expressed? 8. What is the percentage of uncertainty in measuring 50 cm3 of water in a 50 cm3 graduated cylinder given that the precision of measurements is 0.2 cm3? Appendix 4 ANSWERS TO DRILL PROBLEMS 1 (b) 500 s (d) 1000 cm3 (f) 6 boxes 2 (b) 2 (d) 3 (f) 5 3 (a) 23.2 (c) 0.022 5 (b) 203.4 cm (d) 0.002034 km. Four significant digits regardless of the units used. 7 0.6%. 116
Appendix 5 Appendix 5: Name
Actinium
Aluminium
Americium
Antmony
Argon
Arsenic
Astatine
Barium
Berkelium
Beryllium
Bismuth
Boron
Bromine
Cadmium
Calcium
Californium
Carbon
Cerium
Cesium
Chlorine
Chromium
Cobalt
Copper
Curium
Dysprosium
Einsteinium
Erbium
Europium
Fermium
Fluorine
Francium
Gadolinium
Gallium
Germanium
Gold
Hafnium
Helium
Holmium
Hydrogen
Indium
Iodine
Iridium
Iron
Krypton
Lanthanum
Lawrencium
Lead
Lithium
Lutetium
Magnesium
Manganese
Mendelevium
117 The elements SYMBOL Z Atomic mass/
amu Ac
Al
Am
Sb
Ar
As
At
Ba
Bk
Be
Bi
B
Br
Cd
Ca
Cf
C
Ce
Cs
Cl
Cr
Co
Cu
Cm
Dy
Es
Er
Eu
Fm
F
Fr
Gd
Ga
Ge
Au
Hf
He
Ho
H
In
I
Ir
Fe
Kr
La
Lr
Pb
Li
Lu
Mg
Mn
Md
89
13
95
51
18
33
85
56
97
4
83
5
35
48
20
98
6
58
55
17
24
27
29
96
66
99
68
63
100
9
87
64
31
32
79
72
2
67
1
49
53
77
26
36
57
103
82
3
71
12
25
101
227.0
26.98
(243)
121.8
39.95
74.92
(210)
137.3
(247)
9.012
209.0
10.81
79.90
112.4
40.08
(251)
12.01
140.1
132.9
35.45
52.00
58.93
63.55
(247)
162.5
(252)
167.3
152.0
(257)
19.00
(223)
157.25
69.73
72.61
197.0
178.5
4.003
164.9
1.008
114.8
126.9
192.2
55.85
83.80
138.9
(260)
207.2
6.941
175.0
24.31
54.94
(258)
Name
Mercury
Molybdenum
Neodymium
Neon
Neptunium
Nickel
Niobium
Nitrogen
Nobelium
Osmium
Oxygen
Palladium
Phosphorus
Platinum
Plutonium
Polonium
Potassium
Praseodymium
Promethium
Protactinium
Radium
Radon
Rhenium
Rhodium
Rubidium
Ruthenium
Samarium
Scandium
Selenium
Silicon
Silver
Sodium
Strontium
Sulphur
Tantalum
Technetium
Tellurium
Terbium
Thallium
Thorium
Thulium
Tin
Titanium
Tungsten
Uranium
Vanadium
Xenon
Ytterbium
Yttrium
Zinc
Zirconium
SYMBOL Z Atomic mass/ amu Hg
Mo
Nd
Ne
Np
Ni
Nb
N
No
Os
O
Pd
P
Pt
Pu
Po
K
Pr
Pm
Pa
Ra
Rd
Re
Rh
Rb
Ru
Sm
Sc
Se
Si
Ag
Na
Sr
S
Ta
Tc
Te
Tb
Tl
Th
Tm
Sn
Ti
W
U
V
Xe
Yb
Y
Zn
Zr
80
42
60
10
93
28
41
7
102
76
8
46
15
78
94
84
19
59
61
91
88
86
75
45
37
44
62
21
34
14
47
11
38
16
73
43
52
65
81
90
69
50
22
74
92
23
54
70
39
30
40
200.6
95.94
144.2
20.18
237.1
58.69
92.91
14.08
(259)
190.2
16.00
106.4
30.97
195.1
(244)
(209)
39.10
140.9
(145)
231.0
226.0
(222)
186.2
102.9
85.47
101.1
150.4
44.96
78.96
28.09
107.9
22.99
87.62
32.07
181.0
(98)
127.6
158.9
204.4
232.0
168.9
118.7
47.88
183.9
238.0
50.94
131.3
173.0
88.91
65.39
91.22
NOTE: Atomic masses in this table are given relative to carbon‐12 and limited to four significant figures, although some atomic masses are known more precisely. For certain radioactive elements the numbers listed (in brackets) are the mass numbers of the most stable isotopes. Ia
1
H
1.008
3
VIII a
Periodic Table of the Elements
2
II a
III a
IV a
Va
VI a
VII a
4
5
6
7
8
9
He
4.003
10
Li
Be
B
C
N
O
F
Ne
6.941
9.012
10.81
12.01
14.01
16.00
19.00
20.18
11
12
13
14
15
16
17
18
Na
Mg
22.99
24.31
19
20
III b
21
IV b
22
Vb
23
VI b
24
VII b
25
VIII b
27
26
Ib
29
28
II b
30
Al
Si
P
S
Cl
Ar
26.98
28.07
30.97
32.07
35.45
39.95
31
32
33
34
35
36
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
39.10
40.08
44.96
47.88
50.94
52.00
54.94
55.85
58.93
58.69
63.55
65.39
69.72
72.61
74.92
78.96
79.90
83.80
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
85.47
87.62
88.91
91.22
92.91
95.94
(98.91)
101.1
102.9
106.4
107.9
112.4
114.8
118.7
121.8
127.6
126.9
131.3
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Cs
Ba
*La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
132.9
137.3
138.9
178.5
181.0
183.8
186.2
190.2
192.2
195.1
197.0
200.6
204.4
207.2
209.0
(209.0)
(210.0)
(222.0)
87
88
89
Fr
Ra
**Ac
(223.0)
(226.0)
(227.0)
58
*Lanthanides
**Actinides
59
60
61
62
63
64
65
66
67
68
69
70
71
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
140.1
140.9
144.2
(146.9)
150.4
152.0
157.3
158.9
162.5
164.9
167.3
168.9
173.0
175.0
90
91
92
93
94
95
96
97
98
99
100
101
102
103
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
(232.0)
(231.0)
(238.0)
(237.1)
(244.1)
(243.1)
(247.1)
(247.1)
(251.1)
(252.1)
(257.1)
(258.1)
(259.1)
(260.1)