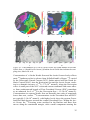
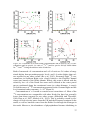
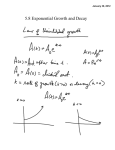
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Iodine Isotopes and their Species in Surface Water from the
Survey
Document related concepts
Raised beach wikipedia , lookup
Indian Ocean wikipedia , lookup
Marine microorganism wikipedia , lookup
Southern Ocean wikipedia , lookup
Sea in culture wikipedia , lookup
Marine life wikipedia , lookup
Ocean acidification wikipedia , lookup
History of research ships wikipedia , lookup
Atlantic Ocean wikipedia , lookup
Marine debris wikipedia , lookup
Ecosystem of the North Pacific Subtropical Gyre wikipedia , lookup
Arctic Ocean wikipedia , lookup
Physical oceanography wikipedia , lookup
The Marine Mammal Center wikipedia , lookup
Marine habitats wikipedia , lookup
Effects of global warming on oceans wikipedia , lookup
Transcript
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1068 Iodine Isotopes and their Species in Surface Water from the North Sea to the Northeastern Atlantic Ocean PENG HE ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2013 ISSN 1651-6214 ISBN 978-91-554-8743-0 urn:nbn:se:uu:diva-206711 Dissertation presented at Uppsala University to be publicly examined in Axel Hambergsalen, Villavägen 16, Uppsala, Friday, October 18, 2013 at 13:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Abstract He, P. 2013. Iodine Isotopes and their Species in Surface Water from the North Sea to the Northeastern Atlantic Ocean. Acta Universitatis Upsaliensis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1068. 73 pp. Uppsala. ISBN 978-91-554-8743-0. Huge amounts of anthropogenic 129I have been and still are released to the environment through liquid and gaseous discharges from the nuclear fuel reprocessing facilities worldwide and in particular the ones in Europe. Most of this 129I signal has been accumulated in the marine environment which plays a major role in the iodine natural pool. In this thesis, an overview of available 129I concentrations in waters of the oceans and marginal seas together with new data about 129I and 127I spatial distribution in surface seawater along a transect between the North Sea and the northeastern Atlantic Ocean are presented. After comprehensive chemical separation, the concentrations of iodine isotopes (127I and 129I) and their species (iodide and iodate) were analysed using accelerator mass spectrometry and inductively-coupled plasma mass spectrometry. The results show that, generally, changes in the 127I and 127I-/127IO3- are comparable to data from other marine waters which are related to natural distribution patterns. A considerable variation of 129I along the transect is observed with the highest values occurring in the eastern English Channel and relatively low values obtained in the northeastern Atlantic Ocean. Inventory estimations of 129I in the North Sea and the English Channel are 147 kg and 78 kg, respectively, where more than 90% resides in the Southern Bight and the eastern English Channel. Iodate is the dominant iodine species for both 127I and 129I in most seawater samples from the North Sea to the Atlantic Ocean. 129I species variability suggests a slow process of 129I- oxidation in the open sea. It takes at least 10 years for the 129I-/129IO3- pair to reach their natural equilibrium as the water is transported from the English Channel. The results suggest a main transport of 129I from the western English Channel via the Biscay Bay into the northeastern Atlantic Ocean. Further, high 129I/127I and distinctive 129I-/129IO3- values south of 40°N indicate possible contribution of 129I through Mediterranean Outflow Water. The environmental radioactive impact of 129I and possible applications in ecosystem studies are also discussed. Keywords: iodine isotopes, 129I, 127I, oceans, North Sea, speciation, Atlantic Ocean, English Channel, Celtic Sea, AMS, iodine chemistry, geochemistry Peng He, Uppsala University, Department of Earth Sciences, LUVAL, Villav. 16, SE-752 36 Uppsala, Sweden. © Peng He 2013 ISSN 1651-6214 ISBN 978-91-554-8743-0 urn:nbn:se:uu:diva-206711 (http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-206711) In memory of my Grandma Zhang, Dedicated to my parents List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I II III IV V He, P., Aldahan, A., Possnert, G., Hou, X. L. (2013) A summary of global 129I in marine waters. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 294:537–541. He, P., Aldahan, A., Possnert, G., Hou, X. L. (2013) Temporal variation of iodine isotopes in the North Sea. Environmental Science & Technology, under review. He, P., Aldahan, A., Possnert, G., Hou, X. L. (2013) Iodine isotopes in the English Channel: a source region of 129I. Manuscript. He, P., Aldahan, A., Hou, X. L., Possnert, G. (2013) Radioactive 129I in surface water of the Celtic Sea. Journal of Radioanalytical and Nuclear Chemistry, in press. He, P., Aldahan, A., Hou, X. L., Possnert, G. (2013) Iodine isotopes species fingerprinting environmental conditions in surface water along the northeastern Atlantic Ocean. Scientific Reports, in press. Reprints were made with permission from the respective publishers. I have written first draft of all papers, with other authors contributing to further interpretations and discussions. I made all the sampling and chemical preparation. I also performed data evaluation, calculation and modeling. Contents 1. Introduction ................................................................................................. 9 1.1 General background .......................................................................... 9 1.2 Objectives of this thesis .................................................................. 11 1.3 Stable iodine (127I) and its sources .................................................. 11 1.4 Radioactive iodine (129I) and its sources ......................................... 12 1.5 Applications of 129I and its species .................................................. 15 2. A short review of iodine (127I and 129I) and its species in natural surface environments ................................................................................................. 18 2.1 127I and 129I in fresh water (rivers and lakes) ...................................... 18 2.2 127I and 129I in seaweed ....................................................................... 19 2.3 127I and 129I in the atmosphere and precipitation................................. 21 2.4 127I and 129I in soil and sediment ......................................................... 22 2.5 127I and 129I in oceans .......................................................................... 23 3. Sampling sites and analytical techniques .................................................. 25 3.1 Sampling sites .................................................................................... 25 3.1.1 2010/2011 Southern Ocean expedition ....................................... 25 3.1.2 The North Sea samples ............................................................... 26 3.1.3 English Channel and Celtic Sea samples .................................... 27 3.1.4 The northeastern Atlantic Ocean samples .................................. 27 3.2 Measurement technique of iodine (127I and 129I) from seawater ......... 30 3.2.1 Sample preparation and chemical reagents ................................. 30 3.2.2 Separation of iodide and iodate from seawater ........................... 30 3.2.3 Extraction of iodine species (127I and 129I) .................................. 30 3.2.4 ICP-MS determination of 127I ..................................................... 32 3.2.5 AMS determination of 129I .......................................................... 32 4. Results and discussion .............................................................................. 33 4.1 Distribution of 129I in global oceans ................................................... 34 4.2 Variations of iodine (127I and 129I) and its species along the 2010 transect ..................................................................................................... 36 4.2.1 Iodine isotopes species in the North Sea .................................... 36 4.2.2 Iodine isotopes species in the English Channel .......................... 38 4.2.3 Iodine isotopes species in the northeastern Atlantic Ocean ........ 41 4.3 Sources of 129I ..................................................................................... 43 4.3.1 Sources of 129I- in the North Sea ................................................. 43 4.3.2 Sources of 129I in the northeastern Atlantic Ocean ..................... 44 4.4 129I inventories in the North Sea and the English Channel ................. 46 4.5 Transformation among iodine species in seawater ............................. 48 5. Environmental impact of 129I .................................................................... 51 6. Conclusions ............................................................................................... 53 7. Acknowledgements ................................................................................... 55 8. Summary in Swedish ................................................................................ 57 9. References ................................................................................................. 60 1. Introduction 1.1 General background Iodine is a trace element which ubiquitously exists in the Earth’s surface environment (i.e. atmosphere, lithosphere, hydrosphere and biosphere). Due to its biophilic nature, iodine is easily taken up by organisms and therefore tends to be enriched in organic matters (Vinogradov, 1953). Iodine is found in high concentrations in marine seaweed and in some brown algae, the concentration by dry weight measurement can reach > 10-3 g·g-1 (Hou and Yan, 1998), while in terrestrial plants, the concentrations are normally lower (Fuge and Johnson, 1986). As a nutrient element, iodine is indispensable and essential to all mammals. Many trace elements in the environment have a close relation to the human health and nutrition. Linkage between iodine deficiency disorders (IDD) such as goiter and intake amount of iodine in human daily diet have evoked enormous attentions to this element. In addition, recent studies on atmospheric chemistry showed that iodine plays a significant role in the depletion of ozone and in aerosol particles for cloud nucleation, which in turn have a direct impact on global climate change (Solomon et al., 1994; O'Dowd et al., 2002; von Glasow, 2006). Although iodine has long been recognized as an important environmental element, data on distribution of iodine in natural environments were generalized and poorly described in early studies. As one of the most important reservoirs, the oceans contain more than 70% of the mobile iodine in the Earth’s surface environment (Wong, 1991; Alfimov, 2005). Iodine in some sparse seawater samples collected in the Atlantic, Pacific, Arctic and Indian Oceans, as well as the Mediterranean and Red Seas, were discussed in the early decades of the last century (Reith, 1930; Barkley and Thompson, 1960). These studies reported a rather constant concentration of total iodine in oceans, but varying proportions of iodate with location and depth. The first extensive survey of iodine and its speciation in the whole Pacific Ocean was conducted by a Japanese group. They found a latitudinal variation of iodide in the surface of the Pacific seawaters and they related this feature to the high biological productivity. In the meantime, they also observed elevated iodide concentration in bottom waters (Tsunogai, 1971; Tsunogai and Henmi, 1971). Later, a systematic investigation of iodine concentration in near surface waters was deployed along a transect across the Atlantic Ocean, which presented a rather similar feature in terms of meridional distribution of io9 dine (including its species) as that observed in the Pacific Ocean (Truesdale et al., 2000). The only long-lived radioactive isotope of iodine, 129I (T1/2 = 15.7 Myr), is mainly introduced into the environment anthropogenically through nuclear activities that started in the 1940s. As a result, the 129I/127I was increased to at least two orders of magnitude higher than the pre-nuclear era globally. Because of its long half-life and low radiation energy, 129I was suggested as a potential tracer of iodine geochemistry (Edwards, 1962). Thereafter some environmental samples were collected for 129I analysis (Oliver et al., 1982; (Fabryka-Martin et al., 1987). The Chernobyl accident, one of the most serious nuclear accidents, occurred on 26 April 1986, and released many types of anthropogenic radioactive isotopes including 129I into the environment. Thus measurements of some soil, rainwater and animal thyroid samples were reported in order to assess the impact of iodine isotopes (129I and 131I) in the vicinity environment of the Chernobyl region (Paul et al., 1987b; Kutschera et al., 1988; VanMiddlesworth and Handl, 1997; Hou et al., 2003; Michel et al., 2005). Release of 129I from the recent Japan Fukushima Dai-ichi nuclear accident on 11 March 2011was estimated by Hou et al. (Hou et al., 2013). Awareness of potential environmental hazards as a result of huge 129I releases from the nuclear fuel reprocessing facilities (NRFs) led to extensive data collection in the surrounding areas of major NRFs such as Hanford (USA), Tokaimura (Japan) (Muramatsu and Ohmomo, 1986), Karlsruhe (WAK, Germany) (Robens et al., 1988), Sellafield (UK), La Hrgue (France) (Edmonds et al., 1998) and Russian facilities (e.g. Mayak, Seversk and Zheeleznogorsk). Estimation of early marine discharges of 129I from La Hague and Sellafield was evaluated by Raisbeck et al. based on times series analysis of 129I/127I in seaweeds (Raisbeck et al., 1995). Since then, many studies have been carried out using 129I as an oceanographic tracer in many 129 I-contaminated regions (e.g. English Channel, Irish Sea, North Sea, Arctic Ocean, etc.) (Beasley et al., 1998; Cooper et al., 1998; Buraglio et al., 1999; Smith et al., 1999; Hou et al., 2000a; Cooper et al., 2001; Edmonds et al., 2001; Alfimov et al., 2004b; Alfimov et al., 2004c; Smith et al., 2005; Hou et al., 2007; Schnabel et al., 2007; Orre et al., 2010; Hansen et al., 2011b; Smith et al., 2011; Yi et al., 2012b; Yi et al., 2013). 10 1.2 Objectives of this thesis The use of chemical tracers (natural and anthropogenic) has expanded our understanding of circulation patterns and ventilation rates in the oceans. Numerous studies have, however, shown that we are still far away from complete understanding of most oceanic processes and further development in tracer technology can, without doubt, substantially increase our knowledge. Investigations of 129I geochemical behavior have been carried out by the Uppsala group for years. These studies were mainly focused on 129I in the marine environment at high latitudes in the Northern Hemisphere, including the Arctic Ocean (Buraglio et al., 1999), the Nordic Seas (Alfimov et al., 2004) and the Baltic Sea (Yi et al., 2010). In the thesis presented here, a focus is given on exploring distribution of iodine isotopes (127I and 129I) and their species (iodide and iodate) along a transect across the North Sea passing by the English Channel and into the northeastern Atlantic Ocean. The objectives of this thesis are: 1. Reviewing of the global distribution of 129I in the oceans and identifying regional variability. 2. Revealing temporal evolution of 129I and its species in surface water of the North Sea in comparison to earlier data. 3. Documenting distribution and sources of iodine isotopes (129I and 127 I) and their species (iodide and iodate) in the surface waters of the English Channel, the Celtic Sea and the northeastern Atlantic Ocean. 4. Exploring the potential of 129I species inter-conversion as a tracer of surface water sources in the northeastern Atlantic. 5. Assessing environmental impact and future effects on the marine ecological systems. Before going into details of the thesis work, a summary of iodine chemistry and distribution in the environment is given below. 1.3 Stable iodine (127I) and its sources Iodine is a redox sensitive element with oxidation states of -1, 0, +1, +3, +5 and +7. There are 37 known isotopes of iodine in which 127I is the only stable one. Although iodine is the least abundant halogen element, it is widespread in Earth’s crust with an average abundance of 0.45 mg/kg (Hou et al., 2009b). Most iodine is found in igneous and sedimentary rocks, though in low concentrations (0.24-4 mg/kg) (Fuge and Johnson, 1986). However, a major part of iodine is bound in rocks, sediments and soils. Only a small 11 amount occurs in the marine pool and the global cycle (Fuge and Johnson, 1986; Moreda-Pineiro et al., 2011). The ocean is the major mobile iodine pool that interacts with land, atmosphere and biosphere. Some studies suggested that the main mode of iodine transport from sea to land is through wet (precipitation) deposition (Truesdalea and Jones, 1996; López-Gutiérrez et al., 2001; Reithmeier et al., 2010). Therefore it seems reasonable to draw a general pattern of decreasing iodine content with increased distance from oceans, which coincides with the prevalence of IDD (Selinus, 2005). However, this hypothesis remains doubtful since some counterexamples have been reported (Krupp and Aumann, 1999). Bearing in mind that iodine is easily incorporated in organic matters and trapped by soils and sediment, distribution of iodine on land is thus also affected by the type of soils (Gerzabek et al., 1999; Yoshida, 1999). In addition, iodine may be derived from erosion of bedrock (Hou et al., 2009b). Emission of iodocarbons from the ocean surface provides a major source of atmospheric iodine which eventually contributes to reactive iodine in the marine boundary layer (MBL). Methyl iodide (CH3I) is considered to be the principal species while recently, high concentration of molecular iodine (I2) was reported in a coastal marine environment (Chatfield and Crutzen, 1990; Saiz-Lopez et al., 2006; Küpper et al., 2008). Accordingly, microalgae (phytoplankton) and macroalgae (seaweeds) appear to play a dominant role in the open sea and coastal areas, respectively (Carpenter, 2003). On land, large iodine emissions from wetlands, vegetation and soils are most important (Sive et al., 2007). Some anthropogenic sources of iodine are related to agricultural and industrial activities. About 5% of methyl iodide in the atmosphere is estimated to be derived from emission of rice paddies (Redeker et al., 2000). The use of soil fumigant in pest control may also add iodine into the environment (Aldahan et al., 2009). Combustion of fossil fuel provides another important source of iodine to the atmosphere duo to relatively high average concentrations of iodine in coal and petroleum, 4 mg/kg and 1 mg/kg respectively (Block and Dams, 1974; Valkovic, 1978). Additionally, application of iodine as a disinfectant makes municipal wastewater treatment plants an important source of iodine in surface waters (Moreda-Pineiro et al., 2011). However, the contribution of iodine from anthropogenic sources inland is much less when compared to natural sources of marine origin and therefore, could be considered negligible (Chameides and Davis, 1980; Carpenter, 2003). 1.4 Radioactive iodine (129I) and its sources The only naturally occurring long-lived (T1/2 = 15.7 Myr) radioisotope of iodine, 129I, which is a beta emitter with relatively low energy radiation (154.4 keV, Eβmax), is an important soluble fission product in spent nuclear 12 fuel. 129I can be naturally produced by spallation of xenon (Xe) induced by interaction with cosmic rays in the upper atmosphere, as well as from spontaneous fission of 238U and thermal neutron-induced fission of 235U inside the Earth (Snyder et al., 2010). Neutron bombardment (130Te) and neutron caption (128Te) of tellurium isotopes are, to a lesser extent, another natural source of 129I (Hou et al., 2009b). The collective 129I natural flux contributes to a 129I/127I ratio of 10-13 to 10-12 prior to human nuclear activities (Broecker and Peng, 1982; Fabryka-Martin et al., 1985; Fehn, 1986; Moran et al., 1998; Fehn et al., 2007) and the total natural inventory of 129I is therefore estimated to be 100-260 kg (Fabryka-Martin et al., 1985; Raisbeck and Yiou, 1999; Snyder et al., 2010), which is insignificant when compared to the anthropogenic sources (Figure 1.1). Since the start of the nuclear era in the 1940s, 129I has been overwhelmingly introduced into the environment by a variety of anthropogenic sources, including: 1) aboveground nuclear weapons tests, 2) nuclear accidents, 3) discharges from nuclear fuel reprocessing plants and possibly 4) the nuclear power plants during routine operation. Aboveground nuclear weapons testing, which peaked in the 1960s, has released various radionuclides into the stratosphere, thus eventually contributing to a global fallout, particularly in the Northern Hemisphere (Wilkins, 1989; Snyder and Fehn, 2004; (Reithmeier et al., 2006). These tests have introduced 57-135 kg of 129I into the environment (NCRP, 1983; Wagner et al., 1996; Raisbeck and Yiou, 1999). Amounts of 129I released by some nuclear accidents are difficult to estimate, but it is considered insignificant, since no evidence of any enhanced 129I has been found in surrounding areas of Windscale (10 Oct. 1957) (Gallagher et al., 2005) and Three Mile Island (28 Mar. 1979) (Moran et al., 2002b; Gallagher et al., 2005). Estimations of 129I emitted by the most serious nuclear accident ever, the Chernobyl accident (26 Apr. 1986), is between 1.3 and 6 kg, which is even 10 times lower than releases from nuclear weapons testing (Paul et al., 1987; Aldahan et al., 2007b). Comparable releases of 129 I (1.2 kg) was estimated from the recent nuclear accident in Japan (12 Mar. 2011, Fukushima) (Hou et al., 2013). 129I release from the nuclear power plants is insignificant (Hou et al., 2002; He et al., 2010). In addition, because of the highly mobile and soluble nature of iodine, dumping of nuclear radioactive waste could be another potential 129I source to the marine deep layer. However, previous surveys on radioactive waste dumping sites in the Atlantic and Pacific Oceans did not show any abnormal concentration of 129I that is above the expected background natural level (Povinec et al., 2000; Cooper et al., 2001). Thus at present, 129I from radioactive waste dumping source is considered negligible. It is worth mentioning that an estimated 68 tons of 129I were produced in routine nuclear power plants operation, mostly waiting for future reprocessing (Hou et al., 2009b). 13 Figure 1.1: Sources of 129I (% inventory) in the environment based on available published literatures from (Aldahan et al., 2007; He et al., 2013; Hou et al., 2009b; Snyder et al., 2010) and references therein. The function of the nuclear reprocessing facilities (NRFs) is to extract fissionable plutonium (Pu) from irradiated nuclear fuel since the 1940s. Some of them were decommissioned (e.g. Hanford and Marcoule) and some are still operating (e.g. Sellafield and La Hague) for commercial use. These NRFs have discharged more than 95% of total 129I inventory into the Earth’s surface environments (Figure 1.1). Among them, around 90% of 129I is released from the two major European NRFs Sellafield and La Hague, which are located in the United Kingdom and France, respectively. Until 2011, these two NRFs have together discharged 5400 kg and 250 kg 129I directly to marine environment and the atmosphere, respectively (He et al., 2013; AREVA; Monitoring our Environment, Sellafield Annual Report). The discharge rate of 129I from these two facilities increased and remained high after 1990 and peaked in 1997, but declined slightly during the past 10 years (Figure 1.2). 14 Figure 1.2: Annual marine and atmospheric releases from (a) Sellafield (UK) and (b) La Hague (France) between 1966 and 2011 (literatures refer to the text). Marcoule (France) is another major European NRF that has released 180 and 75 kg of 129I to the atmosphere and Rhone river, respectively (Hou et al., 2009b). For comparison, only 1.1 kg of 129I was released from the Karlsruhe NRF (WAK), which is located in Germany and was decommissioned in 1987 (Robens et al., 1988). The largest gaseous releases of 129I were from the Hanford NRF (USA), ranging at 300 kg during its operation from 1944 to 1988 (HHIN; Moran et al., 1998). This discharge is even greater than that of the total amount by Sellafield and La Hague. Furthermore, total gaseous 129I released from Savannah River NRF and some major former Soviet NRFs was estimated by an atmospheric transport box model (Reithmeier et al., 2010). The outcome suggested that about 41 and 215 kg of 129I were emitted to the atmosphere during their operation times for Savannah River NRF and former Soviet NRFs (including Mayak, Seversk and Zheleznogorsk) respectively. In Asia, a Japanese NRF located at Tokai, has released about 1.0 kg of 129I to the environment (JAEA; Shinohara, 2004). Release records of the other Asian NRFs (i.e. in China and India) are, however, not well documented. 1.5 Applications of 129I and its species Implementation of the highly sensitive analytical technique of accelerator mass spectrometry (AMS) allowed determination of ultra-low 129I/127I values in pre-nuclear era materials. The present detection limit of AMS and the long half-life of 129I have made the technique a powerful tool in radiometric dating of geochronology within a range of 80 Myr (Nichols Jr et al., 1994; Fehn et al., 2000). In environmental sciences, 129I can be used to study the biogeochemical cycle of stable iodine (Santschi and Schwehr, 2004; Snyder and Fehn, 2004; Schwehr et al., 2005; Karcher et al.). Duo to high releases from nuclear acci15 dent, 131I is one of the most important and harmful radionuclides to the human health. However, it soon becomes undetectable in the environment due to its short half-life (T1/2 = 8 days). Therefore 129I can help to reconstruct 131I dose in order to evaluate 131I deposition patterns after nuclear accidents (Schmidt et al., 1998; Michel et al., 2005). It is also an important radionuclide to assess the safety of radioactive waste repositories. In view of the highly soluble and mobile nature of iodine, 129I could be used as a good tracer in hydrogeological studies. Applications in this field include the tracking of surface water movement processes from infiltration to subsurface flow, investigating the sources and migration characteristics of groundwater, assessment of interactions between surface water and groundwater, as well as estimating age and residence time of groundwater in recharge areas (Fabryka-Martin et al., 1985; Fabryka-Martin et al., 1987; Lehmann et al., 1993; Moran et al., 1998). The sample volume needed for 129 I analysis is relatively small and the isotope often has a greater magnitude of anthropogenic signal than any other traditional tracers (e.g. 137Cs, 3H) in hydrological research (Santschi et al.). As a highly conservative tracer that maintains a strong and consecutive signal in seawaters, 129I became a potential tracer in oceanography. Variations of 129I concentration and 129I/127I ratio in oceans clearly reflect movement and pathways of currents, circulation, mixing and exchanges of water masses, as well as the transport and diffusion patterns of anthropogenic pollutants (Karcher et al., 1998; Smith et al., 1999; Hou et al., 2007; Schnabel et al., 2007; Orre et al., 2010). Time series analysis of 129I/127I and comparison of available release functions from specific NRFs, the early discharge records could be established (Raisbeck et al., 1995; Raisbeck and Yiou, 1999; Gallagher et al., 2005; Reithmeier et al., 2006). In addition to being a retrospective tracer, 129I can also be applied to obtain information of transit time and transfer factor (TF) of contaminants (Smith et al., 1998; Raisbeck and Yiou, 1999; Smith et al., 1999; Hou et al., 2000a; Smith et al., 2005; Keogh et al., 2007; Smith et al., 2011). Speciation analysis of 129I provides a powerful tool for gaining a detailed picture of sources and exchange of water masses in the ocean compared to using total 129I alone. Moreover, speciation of both isotopes offers additional information by identifying redox rates of I-/IO3- pairs in environmental conditions. The huge discrepancy between iodide/iodate for 127I and 129I reflects different sources of iodine isotopes. These potentials have been successfully applied in the North Sea and the Baltic Sea (Hou et al., 2001; Hou et al., 2007; Hansen et al., 2011b; Yi et al., 2012). Because iodine exhibits different behaviors (i.e. mobility and bioavailability) in terms of species in the environment, speciation of 129I in soil, atmosphere and biological samples is therefore helpful in understanding the stable iodine (127I) cycle. However, determination of the individual species of 129I is difficult and thus speciation analysis of 129I is mainly focused on the determination of different 129I16 bounded fractions in these samples (Wershofen and Aumann, 1989; Hou et al., 2000c; Hou et al., 2003). Continuous release of 129I from NRFs and the tremendous amount of it (up to 70 tons) that is waiting for reprocessing makes 129I a source of potential environmental hazard in the long run. Therefore, it is important to monitor the levels of 129I in order to evaluate and predict the consequences for both the environment and humans, since 129I is taken up by organisms and eventually enters the food chain. 17 2. A short review of iodine (127I and 129I) and its species in natural surface environments 2.1 127I and 129I in fresh water (rivers and lakes) Concentrations of 127I in rivers and lakes are not fully investigated and the available data are scarce, scattered and vary widely (0.01-73 ppb, commonly at a range of 0.1- 20 ppb) (Fuge and Johnson, 1986; Moran et al., 2002a). As the main contributor of iodine is expected to be rainfall, concentration of 127I in non-marine surface waters varies with respect to its locations and local soil types, with a mean value of 5ppb. Oceanic iodine is delivered to lakes and rivers through dry/wet deposition and sea-spray. This branch of the iodine cycle tends to lift iodine level in its surrounding fresh water system. Another source is land-derived, by weathering of soils and rocks (Oktay et al., 2001). Surface waters with high content of iodine were also reported in drainage from industrialized and urban rather than rural areas (Whitehead, 1979). Other sources such as vegetation volatilization, degradation of organic matter and groundwater disturbance are, however, regarded as minor fractions (Moran et al., 2002b). Iodide and dissolved organic iodine (DOI) are the predominant species in surface fresh waters with generally high iodide concentration in estuaries (Smith and Butler, 1979; Oktay et al., 2001; Schwehr and Santschi, 2003). However, high iodate was reported in some lake waters, which contradict traditional points of view (Jones and Truesdale, 1984). Interconversion between iodine species is suggested to be driven by biological activities in lakes, but this redox process is not pronounced when compared to some marine waters (Jones and Truesdale, 1984). Variation of 129I in surface fresh water is even larger than that of 127I, with the concentrations of 129I spanning from 106 to 1010 atoms/L and 129I/127I ranges between 10-11 and 10-5 (Snyder et al., 2010). However, the concentration of 129I can be even lower than 106 atoms/L in areas remote from the anthropogenic effects. High 129I concentrations appeared in fresh water systems of Europe and North America with a typical range of 108-109 atoms/L (10-7-10-5 for 129I/127I), suggesting that the influence of NRFs is already evident in continental surface water bodies (Beasley et al., 1997; Rao and Fehn, 1999; Cochran et al., 2000; Buraglio et al., 2001b; Moran et al., 2002a; Keogh et al., 2010). Since a huge amount of 129I has been introduced into the marine system, oceanic re-emission of 129I could be another important source 18 in terrestrial waters. Positive correlations were found for 129I/127I in lake waters (collected in the English Lake District) as a function of distance from Sellafield/Irish Sea, which implied a strong influence by both Sellafield and the Irish Sea (Atarashi-Andoh et al., 2007). Rapid temporal variation of 129I in English and Irish rivers was a response to the change of gaseous release from Sellafield NRF, while this change was not pronounced in rivers far distant from NRFs, such as in Sweden (Buraglio et al., 2001b). Nevertheless, atmospherically transported 129I from Sellafield NRF was also regarded as a major source of 129I in Swedish lakes, where concentrations of 129I was up to 1.4×109 atoms/L and a strong latitudinal dependence was observed (Kekli et al., 2003). Contribution of 129I from the Chernobyl accident seems to be insignificant in relation to elevated 129I level in European lakes, even for those located in the areas of high Chernobyl fallout (Buraglio et al., 2001a). Similarly, local power plants are also believed to have limited influences (Rao and Fehn, 1999; Hou et al., 2002). Considering the fact that iodine easily attaches to suspended particles or binds to organic materials, regional properties such as watershed characteristics (e.g. river flow rate, evapotranspiration rate, catchment areas and vegetation coverage, etc.) and intensity of agricultural and other anthropogenic activities may be involved in altering the 129I/127I in rivers (Oktay et al., 2001; Moran et al., 2002b; Kekli et al., 2003), which can be used to trace organic carbon in estuarine waters (Schwehr et al., 2005). Compared to the dramatic 129I value of ~ 1010 atoms/L found in the Savannah River (USA; Moran et al., 2002a), the 129I levels in surface waters of the southern hemisphere, which is far from the point sources, are 2-4 orders of magnitude lower (106-108 atoms/L, 129I/127I > 10-11) (Snyder and Fehn, 2004), indicating spread of anthropogenic 129I on a global scale, even in remote Antarctica. This anthropogenic 129I in the southern hemisphere includes not only bomb fallout, but also NRF signals from the northern hemisphere. It seems that, however, the distribution of 129I in rivers and lakes is not uniform, but latitudinal-dependent (Fehn and Snyder, 2000). Higher levels of 129I/127I in middle latitude of both hemispheres compared to equatorial and polar regions are probably associated with atmospheric circulation coupled with precipitation patterns on a global scale. Local ecology and marine productivity are also important factors that affect the terrestrial distribution of 129I/127I worldwide. 2.2 127I and 129I in seaweed Iodine can be concentrated in seaweeds with a concentration factor (CF) as high as 106 in some species (Hou et al., 1997), and thus is an ideal bioindicator that is sensitive to its surrounding environment. Concentration of iodine in seaweeds is often higher than inland plants (< 10-6 g·g-1) 19 (Rucklidge et al., 1994). This feature might indicate that seaweeds are more efficient in building up iodine than terrestrial plants, or can be related to difference of iodine speciation between land surface and seawaters. Iodine concentration in seaweeds varies remarkably (10-5-10-3 g·g-1 (dry weight)) and it is reported to be species- and seasonal-dependent, but normally at the level of 10-4 g·g-1 (dry weight) (Hou et al., 1997; Hou and Yan, 1998; Osterc and Stibilj, 2008). Similarly, iodine speciation in marine algae shows a tremendous difference in relation to species of algae. More than 99% of iodine in brown algae is water-soluble, while in some green algae it is < 10%. Further, > 60% and < 40% is iodide and organic iodine in the aqueous leachate, respectively, and the iodate is the least component (< 5%) (Hou et al., 1997). The iodine of water-insoluble form, is mainly bounded with protein, and a lesser content with pigment and polyphenol (Hou et al., 2000c). This chemical speciation of iodine in seaweeds is important as it is linked to bioavailability and biological toxicity. It is suggested that comparable iodine isotopes (129I and 127I) are taken up and assimilated by algae. Therefore 129I/127I in seaweed is usually used as a biological indicator that reflects local level of 129I in seawaters. Additionally, 129 127 I/ I in combination with 129I/137Cs and 129I/99Tc are commonly applied to trace the geochemical cycle of 129I temporally and spatially (Cooper et al., 1998; Hou et al., 2000a; Nies et al., 2010). Because of the sensitive response of 129I level in seaweed to 129I release from NRFs, time series of 129I in seaweeds samples were successfully applied as an oceanographic tracer to reconstruct the missing release record of NRFs in early stages of operation. Furthermore, this approach was coupled with investigations of the ocean current circulation and estimate of transit times, as well as the initial 129I/127I ratio prior to anthropogenic nuclear activities (Raisbeck et al., 1995; Kershaw et al., 1999; Raisbeck and Yiou, 1999; Yiou et al., 2002; Fehn et al., 2007; Keogh et al., 2007; Hou et al., 2009a). 129 I concentration in seaweeds varies from 108 to 1012 atoms/g (dry weight) and depends on species and sampling locations, while 129I/127I in marine algae has increased by 3-4 orders of magnitude from ~10-10 to 10-6 in the last two decades. This increase is mainly due to the increase of 129I discharges from NRFs since the 1990s. The highest levels were of course found in the coastal waters of the Irish Sea and the English Channel, where the 129I/127I in seaweed was >10-5 (Raisbeck et al., 1995; Frechou and Calmet, 2003; Keogh et al., 2007). Other relatively high levels (10-8-10-7) were reported from Denmark and the Norwegian coast, which are apparently contaminated by 129 I plumes from the Sellafield and La Hague advected water towards the north (Hou et al., 2000a; Yiou et al., 2002). In areas remote from the NRFs, which are less or indirectly influenced by 129I-contaminated marine currents, the 129I/127I in seaweeds is in the order of 10-9 (Hou et al., 2000a; Keogh et al., 2007; Osterc and Stibilj, 2008). Extremely low 129I/127I levels of 10-10 or less were measured in Japan, China and USA, as well as the Beaufort and Bering 20 Seas in the Arctic Ocean. These regions are believed to be less affected by releases from Sellafield and La Hague thus the 129I/127I in seaweeds are therefore close to the global fallout level (Kilius et al., 1994; Tseng and Chao, 1996; Cooper et al., 1998; Hou et al., 2000b). 2.3 127I and 129I in the atmosphere and precipitation In the atmosphere, iodine exists as particle/aerosol associated, inorganic gaseous (e.g. I2, HI, HIO, etc.) and organic gaseous (CH3I, CH2I2, etc) substances. Low concentrations of 129I were found in the atmosphere with an order of 104-105 atoms/m3 (10-8-10-9 for 129I/127I ratio) (Santos et al., 2005). The long time needed for 129I and 127I to approach their equilibrium combined with the long time needed to approach their isotopic equilibrium, suggest a different distribution of 129I and 127I speciation in atmosphere (Wershofen and Aumann, 1989). In general, for both 129I and 127I, gaseous organic iodine is the predominant form in the atmosphere, followed by the gaseous inorganic form, while the particle associated iodine content comprises normally < 20% (Wershofen and Aumann, 1989; Hou et al., 2009b; Michel et al., 2012). Furthermore, for 129I, the closer to the NRFs, the higher the content of the gaseous organic form, which is not observed for 127I (Hou et al., 2009b) and photochemical processes were suggested to change the chemical forms of atmospheric iodine. 129I/127I values in all iodine species were at a similar level (~ 10-7). Differently, the highest 129I/127I was measured in particle associated form, and in organic and inorganic gaseous forms, 129 127 I/ I were 3.1 and 1.2×10-7, respectively (Michel et al., 2012). Particle associated iodine is suggested to play a role in cloud formation. High IO3-/Iwas found in oceanic-derived aerosols (1.5-3.0), which was attributed to the accumulation of iodate, as well as to the depletion of iodide depending on aerosol pH (I- + HIO + H+ = IX↑ + H2O) (Vogt et al., 1999; Baker, 2004). However, the rate of this reaction, as well as the formation of iodate was suggested to be slow since a significant proportion of iodide was observed in precipitation (Baker et al., 2001). Available data on concentration of 129I in precipitation varies temporally and spatially (~ 107-1010 atoms/L) while 127I is normally around 1-2 μg/L. In rainwater, iodide is the major specie for 129I while 127I mainly exists as iodate (Hou et al., 2009a; Lehto et al., 2012). In general, concentrations of 129I in rain waters were observed to be higher in coastal areas compared to inland, and are strongly determined by whether the air masses have passed the NRFs areas before precipitation (Bachhuber and Bunzl, 1992; Aldahan et al., 2009; Keogh et al., 2010). In addition, it seems that the concentration of 129I could be continuously lowered by washing out during a rainfall event (LópezGutiérrez et al., 2001; López-Gutiérrez et al., 2004). Extremely high 129I concentrations of > 1010 atoms/L were found in Germany and Ireland, im21 plying a significant influence by atmospheric release of 129I from NRFs, nuclear accidents, as well as the influence from highly 129I-contaminated ocean water (Paul et al., 1987; Krupp and Aumann, 1999b; Keogh et al., 2010). It is suggested that, re-emission of 129I from the ocean surface is the major source that contributed to 129I of rainwater in the European continental coast areas (Hou et al., 2009a). In most areas of Europe, 129I in precipitation is in the order of 108-109 atoms/L (~ 10-7 for 129I/129I) without any specific correlation to latitude (Buraglio et al., 2001b; Persson et al., 2007; Reithmeier et al., 2005), implying a large coverage of NRFs influences. Moreover, 129I released into the atmosphere from NRFs has enough time to travel over long distances based on a suggested residence time of 10-18 days in the atmosphere (Duce et al., 1963), and thus provides a significant source of 129I in regions far away from those directly influenced by Sellafield and La Hague. Rainwater samples collected from USA demonstrate the possible influence from Europe, although the concentrations were 1-2 orders of magnitude lower (Oliver et al., 1982; Moran et al., 1999; Rao and Fehn, 1999). 2.4 127I and 129I in soil and sediment Iodine in soil mainly originates from the sea after wet/dry deposition of atmospheric iodine, or by weathering of rocks. Concentrations of iodine in soils are suggested to decrease with increasing distance from the coast, although some controversies about this issue exist (Fuge and Johnson, 1986; Moran et al., 2002a). Iodine concentration in European surface soil varies at 1-10 μg·g-1 which is affected by rock/soil types, hydrological conditions and land use practices. Eh/pH conditions may play a major role in iodine distribution since iodide tend to volatilize in acid soils, while iodate is more stable in alkaline soils (Rucklidge et al., 1994). Soil is the largest 129I reservoir of the terrestrial environment. Most 127I resides in the top 0-50 cm depth, and 129I is primarily retained within the upper 10 cm at the order of 107-1010 atoms/g (10-9-10-7 for 129I/129I) in Europe (Ernst et al., 2003; Michel et al., 2005; Hansen et al., 2011). Because of the injection of anthropogenic 129I from major NRFs, surface soils in most adjacent areas are contaminated (Muramatsu and Ohmomo, 1986; Rao and Fehn, 1999). High levels of 129I were observed in many samples of European soil which is influenced by Sellafield and La Hague. Besides, sources of Chernobyl fallout is evident in surface soils collected from Ukraine, Belarus and Russia (Hou et al., 2003; Michel et al., 2005). Soil depth profiles show different depth dependencies of the 127I and 129I concentrations and exponential decrease was observed for both isotopes, though the slope of 129I was much steeper in upper 25 cm (Ernst et al., 2003). 22 127 I in the top part of sediments is generally 5-10 times higher than that in soils (> 10 μg·g-1) (Englund et al., 2008; Hansen et al., 2011; Osterc and Stibilj, 2012; Qiao et al., 2012). 129I/127I in lake sediments and marine sediments varies considerably and can provide a time marker for 129I emissions and depositions (Moran et al., 1998; Gallagher et al., 2005). Inventories of iodine in sediments varies a lot depending on sorption coefficients, dynamic sedimentation and erosion processes (Snyder et al., 2010). In many 129Icontaminated seas, accumulated 129I was found in the top 5-10 cm of marine sediments in the range of 109-1011 atoms/g (Wilkins, 1989; López-Gutiérrez et al., 2004a; Aldahan et al., 2007b). However, in marine sediments collected off the USA coast that are remote from European NRFs, the 129I/127I is in good agreement with the pre-nuclear era natural level (Fehn, 1986; Moran et al., 1998). Sequential extraction is widely used in fractionation analysis of iodine in soil and sediment samples and a similar distribution of 129I in soil and sediment is observed (Hou et al., 2003). Generally, around 80% of 129I is bound to oxides and organic matter, and less than 10% of soil leachate are water soluble and exchangeable fractions (Hou et al., 2003; Hansen et al., 2011; Qiao et al., 2012). An exception was reported in soil collected in Germany, where ~ 50% of readily available 129I form was observed, which was attributed to specific soil properties and chemical speciation of deposited 129I (Schmitz and Aumann, 1995). In the organic fraction, 129I was mainly associated with humic and fulvic acids. In addition, it is suggested that the anoxic/oxic conditions may influence the mobility of soil iodine (Hansen et al., 2011) as well as the iodine speciation in the water soluble fraction (Yuita, 1992). For 127I, though the major fraction is bound to oxides and organic matter, the composition is different when compared with 129I because a significant fraction of 127I is not extractable. 2.5 127I and 129I in oceans The ocean is the major iodine reservoir and concentration of 127I in seawater is generally rather uniform (~ 60 ppb). The initial natural ratio of 129I/127I introduced into the ocean is estimated to be 1.5×10-12 without spatial difference, in theory, due to much longer residence time of iodine (30,000 yr) than seawater turnover time (1000 yr) (Broecker and Peng, 1982; Fehn et al., 2007). However, because of human nuclear activities since the 1940s, more than 6000 kg of 129I have been introduced into the environment, leading to dramatic increase of 129I in the world oceans. Nuclear fuel reprocessing is regarded as the major anthropogenic iodine contributor and releases from those facilities dominate the 129I inventory nowadays. Sellafield (UK) and La Hague (France) are the main point sources of 129I, and together they account for 90% of total global emissions. The temporal and spatial variability of 23 anthropogenic 129I is strongly linked to the major point sources in the Irish Sea and the English Channel and the global marine spreading pathways are partly outlined from these sources. However, most of the available 129I data are assembled from small territories and there is no comprehensive picture about the magnitude of 129I distribution in different parts of the world oceans or with respect to water depth (Schink et al., 1995; Carmack et al., 1997; Beasley et al., 1998; Buraglio et al., 1999; Cooper et al., 2001; Smith et al., 2005; Suzuki et al., 2008; He et al., 2010; Povinec et al., 2010). A review of the 129I variability in the world oceans is compiled in paper I of this thesis and is presented in section 4.1. 24 3. Sampling sites and analytical techniques 3.1 Sampling sites 3.1.1 2010/2011 Southern Ocean expedition The 2010/2011 Antarctica two-ship expedition was an international scientific cruise jointly funded by the Swedish Polar Research Secretariat and the US National Science Foundation (NSF). This Antarctica scientific expedition was devoted to studies of physical and chemical oceanography, sea ice, the carbon cycle, the dynamics of Amundsen Sea Polynya, and ecosystems. Investigation of iodine was one of the expedition’s projects and was conducted through a joint cooperation between the Uppsala group (Department of Earth Sciences and The Tandem Laboratory) and the Danish group at the Center for Nuclear Technologies, Technical University of Denmark, Risø Campus. The aims of the project are focused on the distribution of the iodine isotopes (129I and 127I) and species of iodine (I- and IO3-) along the expedition transect. During October-December 2010, the Swedish icebreaker Oden sailed from Landskrona (Sweden) to Punta Arenas (Chile) via the North Sea, the English Channel and the Atlantic Ocean (Figure 3.1). Surface water samples along the first transect of the expedition (North Sea - Canary Islands) represent the material for this thesis. Surface water samples were continuously collected through the Teflon pipes at the Oden water lab. The water samples were planned for analysis of tracer elements, such as iodine, uranium and cesium. Meanwhile, other sampling devices (i.e. Denuder sampler, PM 2.5 sampler, etc.) were set up onboard to collect air samples along the cruise from Sweden to South America, which were aimed for the investigation on organic and inorganic halogen species in the marine boundary layer (MBL). Further, a standard CTD sampler was launched to automatically measure water temperature and salinity along the transect. Meteorological parameters such as wind velocity and humidity were also measured at the same time. Atlantic surface water was continuously sampled at varying sampling density and volume depending on location and expected 129I concentration. Sampling strategy along the Oden route from north to south (Figure 3.1) varied depending on expected variability of 129I concentration in the different water bodies. In addition, the volume of water samples was gradually in- 25 creased from 0.5 L to 2.0 L along the transect in response to the expected 129I concentration in the water. Figure 3.1: Atlantic transect of icebreaker Oden from Sweden to Chile. Different colors represent different strategies of surface seawater sampling. Sampling at each 5 km with 0.5 L for each seawater sample was implemented within the English Channel (purple). Sampling at each 20 km was performed in the North Sea (red, 1.0 L each sample) and each 50 km in the Celtic Sea and the northeastern Atlantic Ocean (yellow, 2.0 L each sample), while 100 km a part sampling was carried out in the rest of the transect (green, 2.0 L each sample). 3.1.2 The North Sea samples The North Sea is a semi-enclosed shallow continental shelf sea with an average of water depth 90 m and lies between 51°N and 62°N where prevailing westerlies dominant. It connects to the saline Atlantic Ocean through Orkney-Shetland-Norwegian channels in the north and the English Channel in the southwest, while it exchanges with brackish Baltic water via the Danish straits. General cyclonic circulation within the North Sea relates to its strong tidal currents and southwesterly wind, and is modified by specific topography and bathymetry (Howarth and Oceanographic, 2001). In its southern part and the coastal areas of the German Bight where the water is well mixed throughout the year, the depth is less than 40 m. Depth 26 in the central and north parts gradually increases from 40 m to 200 m, while in the Norwegian Trench, northeast, deeper regions occur with the deepest, about 750 m, in the Skagerrak. Therefore in these regions, the water column usually stratifies in May with a mean thermocline of 30 m, in particular in the Norwegian Trench (Richardson and Pedersen, 1998; Howarth, 2001). Due to the relatively high 129I level in the North Sea, only 0.5 L surface seawater was collected at each sampling site for 129I separation and analysis. 3.1.3 English Channel and Celtic Sea samples The English Channel is a narrow shallow passage that separates southern England from the European continent. It connects to the Atlantic Ocean in the west and the North Sea through the Dover Strait in its eastern end. The width of the English Channel narrows gradually from the west to east, while the average depth decreases from 120 m to 45 m. The Celtic Sea is an extension of the European continental shelf that opens to the deep Atlantic Ocean. Its northern and eastern limits are bound by the southern Irish Sea and the English Channel, respectively, while the western and southern parts are bordered by the continental slope. The water is relatively shallow (< 100 m) in its northern portion and increases towards south and west with the longest open boundary normally defined by the 200 m isobathymetry. Saline Atlantic water partly enters the English Channel north of Ushant Island (France) and flows eastwards within the channel. Accordingly, an evident gyre is isolated from the main flow in the Gulf of Saint Malo (Salomon and Breton, 1993). Another fraction of Atlantic water moves north along the coast from the Isles of Scilly to Lundy Island in the Celtic Sea and returns southwestward through the St. George’s Channel. This flow pattern continues along the Irish coast thus a counterclockwise current is suggested (Brown et al., 2003). In addition, a weak residual current flows poleward along the west continental slope (Southward et al., 2005). The currents in the Celtic Sea mainly responds to the variation of wind systems in this region, where in summer the westerly winds dominate while wind direction is more southwesterly in winter (Pingree and Le Cann, 1989). It is expected that large variations in 129I concentration occur between the east and west parts of the English Channel. Therefore frequent sampling density was applied within the English Channel, in all 34 samples of 0.5 L each. A small number of samples, only six, were collected in the Celtic Sea, with a volume of 1.0 liter each. 3.1.4 The northeastern Atlantic Ocean samples Although in general the surface ocean waters flow eastward, the whole region of the northeastern Atlantic Ocean is considered to be stagnant (Pingree, 1993). This region is located between subpolar and subtropical gyres where 27 seasonal and regional variations of wind systems in this transition zone (between westerlies and trade winds) highly influence the surface circulation pattern in most parts of the sampling transect. A branch of the North Atlantic Current (NAC) borders the transect in the north, while the southern limit is influenced by a combination of the Azores, Portugal and Canary Currents systems, as well as the injection of Mediterranean Outflow Water (MOW) (Figure 3.2). Figure 3.2: General bathymetric chart of the investigated area in the northeastern Atlantic Ocean. (a) Sampling transect of 129I along the northeastern Atlantic Ocean. Sampling locations are expressed as black dots. Location of Sellafield and La Hague nuclear reprocessing facilities (NRFs) is highlighted with stars. (b) General scheme of surface water circulation in the Bay of Biscay and (c) the Gulf of Cadiz. NAC=North Atlantic Current, IPC=Iberian Poleward Current, PC=Portuguese Current, ACN=Azores Current, northern branch, ACS=Azores Current, southern branch and CC=Canary Current. Intensive in situ hydrographic data and sea surface temperature (SST) satellite observations, as well as numerical models reveal that the eastward Azores Current, combined with the equatorial Portuguese Current that flows parallel to the west Iberia coast, enters the Gulf of Cadiz along the continental slope towards the Strait of Gibraltar. The easternmost part of this anticyclonic circulation eventually feeds the Canary Current. This general fea28 ture of circulation pattern is thought to be weakened in winter and enhanced in summer (Johnson and Stevens, 2000; Relvas and Barton, 2002; Sánchez and Relvas, 2003). Saline and dense MOW mixed with overlying North Atlantic Central Water (NACW) descends as the Mediterranean Undercurrent in the Gulf of Cadiz along the continental slope and interacts with bottom topography. High velocity Mediterranean Water arising from the release of potential energy makes this region highly unstable (Baringer and Price, 1999). On its way to Cape St. Vincent, the plume becomes neutrally buoyant and divides into two parts with their main cores lie at 800 m and 1200 m depth (Zenk, 1970). These Mediterranean waters can be traced across the North Atlantic Ocean along 40°N and even extend to the south of the Iceland-Scotland Ridge (Reid, 1978). Ambar (Ambar, 1983) also identified a shallow Mediterranean core of 400-700 m depth, stretching from the vicinity of the Strait of Gibraltar to the area off the Cape Roca of the western Portugal coast. Mauritzen et al. (Mauritzen et al., 2001) make a point that a salinity detrainment process (towards low-density levels fluxes) occurs in the eastern Gulf of Cadiz, which partly results in a higher salinity of East North Atlantic Central Water compared to that in the west. Many models and direct observations show that the Atlantic Water not only flows eastward towards the Mediterranean Sea, but also has a recirculation within the upper layers in the Gulf of Cadiz (Johnson and Stevens, 2000; Mauritzen et al., 2001; Machín et al., 2006) (Figure 3.2). During winter, an Iberian Poleward Current was observed along the Portugal coast as a recirculation of the Azores Current (AC) which centered at about 35°N (Mazé et al., 1997; Peliz et al., 2005). Although the origin and variability of this slope-flow current is still unclear, it may be connected with the coastal counter flow in the western Cadiz. The main route of this westward flowing Atlantic Water is along the slope of the European continent and it is well defined west of Cape Santa Maria and strengthened around Cape St. Vincent. Thus the modified surface water in the Gulf of Cadiz can subsequently spread westward and northward. Considering long-term currents circulation patterns in the northeastern Atlantic Ocean, as well as the relatively long distance from Sellafield and La Hague, concentration of 129I should be low in this region (31.07-47.36 °N, 7.96-14.52°W). Therefore, the sampling frequency was reduced and the volume of each sample was increased to 2 liters. 29 3.2 Measurement technique of iodine (127I and 129I) from seawater 3.2.1 Sample preparation and chemical reagents All collected samples were instantly filtered onboard through a 0.45 μm membrane (Sartorius AG, Gottingen, Germany) and filled in clean polyethylene containers under cold and dark conditions. 129I standard (NIST-SRM4949c) was purchased from National Institute of Standard and Technology (Gaithersburg, MD, USA). Carrier-free 125I was purchased from Amersham Pharmacia Biotech (Little Chalfout, Buckinghamshire, UK) and carrier 127I (Woodward iodine) from MICAL Specialty Chemicals (New Jersey, USA). Bio-Rad AG1- ×4 anion exchange resin (50-100 mesh, Cl- form, Bio-Rad laboratories, Richmond, CA) was used for iodine separation because of different affinities of iodide and iodate on the resin. All chemical reagents used were of analytical grade and all solutions were prepared using deionised water (18.2 MΩ·cm-1). A large column was loaded with AG1- × 4 anion exchange resin (50-100 mesh, in Cl- form) and washed with 300 ml of 2 mol/L NaNO3 until Cl- ion was washed off from the resin. Afterwards the column was washed with 50 ml deionised water. Then the prepared converted resin (in NO3- form) was transferred to a column (Ø 1.0 × 20 cm) for separation of iodine. 3.2.2 Separation of iodide and iodate from seawater A method for separation of iodine species (iodate and iodide) developed by Hou et al. (Hou et al., 2001) was used in the experiment (Figure 3.3). The filtered seawater, mixed with 0.1 ml of 125I- tracer (250 Bq), was loaded to the prepared AG1- × 4 NO3- form column (Ø 1.0 × 20 cm) with a flow rate 2-4 ml/min. After the seawater pass through, the column was washed with 30 ml deionised water and followed by 50 ml of 0.2 mol/L NaNO3. The effluent was collected for solvent extraction of iodate. The iodide (including 125 I ), however, is adsorbed onto the resin, and can be eluate with 60 ml of 10% NaClO solution. The eluate is used for iodide extraction. Meanwhile, 1.0 ml of the effluent seawater, as well as the eluate was used for determination of 127I using ICP-MS (Inductively Coupled Plasma Mass Spectrometry). 3.2.3 Extraction of iodine species (127I and 129I) To extract iodate and total inorganic iodine, 1.0 ml of 127I carrier (Woodward iodine, 2 mg/ml) and 0.1 ml tracer (125I-, 250 Bq) were added to the filtered seawater and the effluent of the iodate fraction. In order to reduce all the iodine to iodide form, 10 ml 3 mol/L HNO3 and 1.0 ml of 1 mol/L Na2S2O5 were added in turn, and the pH was maintained at < 2 to ensure a fast reduc30 tion. Then the mixed solution was transferred to a suitable separation funnel with addition of 20-50 ml CHCl3. Iodide was oxidized to iodine (as I2) by using 2-5 ml of 1.0 mol/L NaNO2 and extracted to the CHCl3 phase. This procedure is repeated two times to extract all iodine to organic phase. 0.5 ml of 3 mol/L HNO3 is added to the funnel to provide enough hydrogen ions before oxidizing iodide in each extraction. The extraction of iodate from the eluate is much the same. The differences are that we use a 1.0 mol/L NH2OHHCl solution to reduce iodate to molecular iodine. Finally, 0.2 ml of 0.05 mol/L Na2S2O5 was added to the funnel and iodine in the organic phase was back-extracted to the water phase, then transferred to a vial to which 0.1 ml of NH3·H2O was added to remain iodide stable before precipitation. The precipitation of AgI was achieved through addition of 1.0 mol/L AgNO3. The AgI precipitate was dried at 70oC. Figure 3.3: Analytical scheme for chemical speciation of 127I and 129I species from seawater. Modified from (Hou et al., 2001). The chemical separation yield is obtained by measurement of 125I in the final separated solution and the 125I added to the solution (as standard) using a NaI 31 gamma detector (well type, Bicron) at channels 25-115 in 26-36 keV for 60 seconds. The chemical yield (Y) is calculated by: Y = (counts of sample) / (counts of 125I standard). The chemical yield of total iodine, iodate and iodide during the separation processes were calculated to be 81-99%, 97-99% and 53-80%, respectively. 3.2.4 ICP-MS determination of 127I Measurement of 127I was performed using X-Series inductively coupled plasma mass spectrometry (ICP-MS) system under hot plasma conditions with Xt interface. Before measurements, each 1.0 mL of raw sample was spiked with Cs+ as internal standard, and then diluted to 20 mL with 1% of ammonium solution. The detection limit for 127I calculated as 3 SD of blanks was 0.05 ng/mL. 3.2.5 AMS determination of 129I The dried silver iodide precipitation (AgI) was mixed with niobium powder (~7.4 mg) in a mass ratio of 1:2. The mixed sample was then transferred and pressed into a copper holder for the AMS measurement in the Tandem Laboratory, Uppsala University. Negative ions beam of iodine is sputtered out from the copper holder and selected by a 90° bouncer magnet. A voltage of 3.5 MV was applied in the terminal to measure 129I. The 129I /127I isotopic ratio in the standard (NIST-SRM-4949C) is (1.1±0.1) ×10-11 and the ultralow background of the AMS system is 4×10-14. The statistical error of the measurements was < 10% (one standard deviation). 32 4. Results and discussion The whole data sets of 129I and 127I and their species along the transect are shown in Figure 4.1 and 4.2. Because of the huge number of data and the large variability in oceanographic characteristics of the transect areas, the data were divided into four sets representing the regions: the North Sea (NS, paper II), the English Channel (EC, paper III), the Celtic Sea (CS, paper IV) and the northeastern Atlantic Ocean (NEAO, paper V). Detailed discussion of each region is given in this section. Furthermore, a review of 129I distribution in the marine waters is given in paper Ⅰand summarized in section 4.1. Figure 4.1: Analytic results of 129I and its species along the transect of Oden Antarctica expedition used in this thesis. NS = North Sea, EC = English Channel, CS = Celtic Sea, NEAO = northeastern Atlantic Ocean. 33 Figure 4.2: Analytic results of 127I and its species along the transect of Oden Antarctica expedition used in this thesis. NS = North Sea, EC = English Channel, CS = Celtic Sea, NEAO = northeast North Atlantic Ocean. 4.1 Distribution of 129I in global oceans The 129I concentration spans from 6.0×106 atoms/L (southern Indian Ocean) to 1.3×1012 atoms/L (Irish Sea) which is 1.3-6 orders of magnitude compared to pre-anthropogenic background value. The Nordic Seas and the Arctic Ocean present the major 129I reservoir, while the Pacific and Indian Oceans have low concentrations close to the nuclear fallout level, reflecting less influence from nuclear reprocessing facilities (Figure 4.3). Highest values (>1×1010 atoms/L) are restricted along the 50-70°N bands and near to the vicinity of two point sources in Western Europe. Relatively higher levels could be also found in the Arctic Ocean and Nordic Seas region where the average 129I is 1×109 atoms/L, about 100 times above nuclear weapons testing fallout value, with a gradual decrease to ~1×108 atoms/L in the northwest of Atlantic Ocean. 129I concentrations in south of 40°N in Northern Hemisphere decrease abruptly, ranging from ~106 to ~108 atoms/L which is generally 2-3 orders of magnitude lower compared to north of 40°N. 34 Figure 4.3: Concentrations of 129I in (a) Arctic Ocean, (b) North Atlantic Ocean and Nordic Seas, (c) North Pacific Ocean, (d) Indian Ocean. Solid dots indicate the sampling sites, data in He et al. Concentration of 129I in the Nordic Seas and the Arctic Ocean clearly reflects main 129I pathways after its release from Sellafield and La Hague. 129I carried by the Norwegian Coastal Current (NCC) further mixed with the North Atlantic Current (NAC) into the Norwegian Sea and the Arctic Ocean, resulting in a dramatic increase of 129I (~3.5×1010 atoms/L) along the Norwegian coast. A small part of the NCC westward current combined with water masses from southeastward branch of East Greenland Current (EGC) contribute to an enhanced level of 129I in the Norwegian Sea (~1.5-10×108 atoms/L). Concentrations in western Nordic Seas are normally one order of magnitude lower than east, surface 129I concentrations in the Greenland Sea and EGC are typically 5-10×108 atoms/L, with higher levels above the eastern Greenland shelf and decrease towards the central Greenland Gyre. Within the Arctic Ocean, the 129I-bearing water enriched in the Barents and Kara Seas moves along the continental margin, with a small component entering the 35 Canadian Basin and undergoing further bifurcation at Chukchi Plateau, whereas a large part returns along the Lomonosov Ridge and flows out of the Arctic through Fram Strait. This near continental high-129I flow seems not to incorporate into the fresher Siberia Coastal Current eastward towards Chukchi Sea because a remarkable 129I gradient is observed, suggesting an encounter of the Pacific- and Atlantic-origin water masses within the Chukchi Sea. 4.2 Variations of iodine (127I and 129I) and its species along the 2010 transect 4.2.1 Iodine isotopes species in the North Sea The concentrations of total iodine in the North Sea range from 0.31 to 0.41 μM with an average of 0.37 μM (Figure 4.4a). Maximum 127I appears in the Southern Bight whereas the distinctly lowest iodine concentration occurs off the coast of the West Frisian Islands, Netherlands. Iodate concentrations generally follow the same pattern as that of total iodine, which decrease to the lowest level of 0.20 μM from the German Bight to the Dutch coast, and then increase up to 0.35 μM in southern seawater samples. Iodide ranges between 0.06 and 0.18 μM in the surface waters, although the average concentration (0.11 μM) is markedly lower than the predominant species of iodate. The highest iodide value is observed close to the Dutch coast. The results of 129I and its species (129I- and 129IO3-) in the North Sea show a rather similar pattern, with a significant increase from 54°N to 52°N (Figure 4.4b). The concentrations of 129I indicate a nearly two orders of magnitude variation along the transect, ranging from 9.21×109 to 2.45×1011 atoms/L with an average of 8.15×1010 atoms/L. Higher values of over 2×1011 atoms/L occurred in the south close to the Dover Strait, whereas the low concentrations around 1×1010 atoms/L was observed in the north part of the sampling campaign, relatively distant from the English Channel. The distributions of 129I species depict a similar behavior with that of total 129I, and the concentrations of 129IO3- (3.2×109-1.5×1011 atoms/L) show a relatively wider range than 129I- (6.9×109-1.2×1011 atoms/L). It is worthwhile to observe that compared to speciation of 127I, iodide is generally the dominant species of 129 I in most samples, except the two in the south. Similar to the pattern of 129I, values of 129I/127I atomic ratio increased by almost two orders of magnitude from north to south (4.4×10-8 to 1.0×10-6) with an average of 3.5×10-7 (Figure 4.4c). For species of 129I/127I, the average levels of 129I-/127I- and 129IO3-/127IO3- are 5.9×10-7 and 2.7×10-7, respectively. The atomic ratios of 129I/127I for iodide along the transect are all higher than that of 127I, as well as iodate, even in southern samples which have relatively high 129I-iodate concentration. 36 Figure 4.4: Variations of (a) total iodine concentrations and its species, (b) 129I concentrations and its species, (c) ratio of 129I/127I and its species and (d) iodide/iodate for 127I and 129I along the transect in the North Sea. Both of measured 129I concentration and 129I/127I ratio are 5-6 orders of magnitude higher than pre-anthropogenic levels, and 1-4 orders higher than values reported in any other oceans (He et al., 2013). Extremely high 129I concentrations in Southern Bight were clearly linked to the English Channel water (one branch of the North Atlantic Water), this water is mixed with the relatively low 129I of the North Sea water and is diluted as the water parcel moves northward along the continental coast in a short distance. A nearly 20-fold decrease of 129I concentrations appeared in the German Bight and the level remained rather constant (~1×1010 atoms/L). Concentrations of 127I varied to a much lesser extent than 129I. Most of the 127 I concentrations are comparable with other North Sea data, but generally lower than those reported in any other oceans (Elderfield and Truesdale, 1980; Hou et al., 2007; Michel et al., 2012). This is probably caused by discharge of fresh riverine water from the UK and the European continent in the south, as well as brackish water from the Baltic Sea through the Kattegat in the north. Moreover, the abundance of phytoplankton biomass inhabiting in 37 this temperate shallow shelf sea, especially in the Southern and German Bights, imply a potential organisms uptake of iodine in the North Sea (Joint and Pomroy, 1993). A positive correlation between salinity and the 127I concentration (r = 0.62, P < 0.05) was observed in all seawater samples investigated here. This feature also suggests that continuous dilution of saline and high iodine content of Atlantic water by continental runoff occurred from south to the north. Compared to total 127I, the species (iodide and iodate) show a considerable variation. The lowest total 127I concentration was observed close to the west Wadden Sea, which might demonstrate a relatively strong influence from the fresher west Wadden Sea (Zimmerman, 1976). This feature is also confirmed by lowest salinity and low iodate content in this sample. 4.2.2 Iodine isotopes species in the English Channel Higher concentration of total iodine was observed in the English Channel compared to that in the Celtic Sea (mean 0.38 μM), particularly in the southwestern part (off Plymouth), where the average iodine concentration reaches 0.48 μM. In contrast, concentrations of iodate is generally increased from east to west along the transect and do not show pronounced variation between the Channel water and the open sea. Although salinity data is not available for some of the samples located in the Celtic Sea, the rationalized iodate (normalized to a salinity of 35) was rather constant (around 0.33 μM) west of La Hague. High iodide and 127I-/127IO3- in the English Channel suggest a more productive water compared to the Celtic Sea, which is confirmed by high concentration of chlorophyll a (Gentilhomme and Lizon, 1997). Insignificant correlation between iodate and iodide (r = -0.34 and P = 0.22) and the nearly constant rationalized iodate concentration (0.33 μM) between the western English Channel and the open sea suggests that, to a large extent, the changes in iodine speciation within this region is not primarily a result of interconversions between iodate and iodide. Higher concentration of total iodine in the western English Channel seems directly linked to the elevation of iodide (r = 0.89, P < 0.0001; slope = 0.8) and thus the net increase of iodine is mainly attributed to direct addition of iodide in this area. However, no similar pattern was observed in 129I and its species. This is because the 129 I is overwhelmingly influenced by the source point of La Hague in the English Channel, as well as a relatively long time for 129I to reach open sea equilibrium. 38 Both 129I concentrations and 129I/127I in the English Channel are 4-5 orders of magnitude higher than pre-nuclear values. The changes of 129I and 129I/127I along the transect show a rather similar trend that is continuously decreasing towards the west (Figure 4.5). There is a ‘great sink’ starting just west of the La Hague tip, as 129I concentrations dramatically drop from ~1010 to ~108 atoms/L. This is attributed to the major Atlantic water pathway in the English Channel, which results in a much more serious contamination of seawater in the North Sea in the east rather than the Atlantic Ocean in the west. Within the English Channel, the highest 129I concentration (4.6×1011 atoms/L) did not occur in samples that are close to the La Hague tip, but in the surrounding area of the Dover Strait. This is due to that the sampling transect was slightly towards the British Isles and the major water movement along the Channel causes dispersion from the French to the British side of the Channel. The same pattern was also reported in studies of other radionuclides such as 125Sb, 134+137Cs and 90Sr (Herrmann et al., 1995; Bailly du Bois and Guéguéniat, 1999). Figure 4.5: Concentration of Shelf in 2010. 129 I in the English Channel and the southern Celtic Similar to 127I, iodate in the English Channel is the dominant species for 129I with a few exceptions, and this observation can generally be extended to the Celtic shelf (Figure 4.6). However, the ratio of iodide to iodate for 129I is quite different when compared with that of 127I. All of the 129I-/129IO3- values 39 were significantly higher than the corresponding 127I-/127IO3-, which may be attributed to the presence of ‘old’ 129I that reached its species equilibrium. This is because the major source of 129I in the English Channel originated from La Hague and thus the observed 129I-/129IO3- should directly reflect the proportion of 129I species in liquid discharges. It is worthwhile to mention that the average 129I-/(129IO3- + 129I-) in the English Channel in 2010 (0.43) is comparable to that in 2005 (0.41), though the data available in 2005 were sparse (Hou et al., 2007). This finding is vital for the estimate of 129I redox rate in marine water as the percentage of 129I- in the source water could be regarded constant. Moreover, there was no significant difference for 129I/(129IO3- + 129I-) in the west and east of the English Channel (P=0.054) in 2010. Despite more than one order of magnitude lower 129I concentration west of La Hague, this feature again confirms that the western English Channel has also significantly been contaminated by the La Hague discharges and that the oxidation of iodide is a slow process within the English Channel. Compared to the data presented here, up to 20 times higher 129I/129IO3- values were reported in the offshore of Fukushima surface water that was contaminated by the Japanese nuclear power plant (NP) accident in March 2011 (Hou et al., 2013). This suggests that a higher 129I-/129IO3- level is always expected in discharges of 129I through anthropogenic nuclear activities and evidently, different NRFs/NPs have different initial 129I-/129IO3values in their source waters. Figure 4.6: Ratio of iodide/iodate for southern Celtic Sea in 2010. 40 129 I and 127 I in the English Channel and the 4.2.3 Iodine isotopes species in the northeastern Atlantic Ocean It is apparent that, as a sensitive radioactive tracer, the concentration of 129I (including 129I- and 129IO3-) shows a larger variation in the seawater compared with that of 127I (Figure 4.7). More than one order of magnitude difference in 129I concentration is shown in the northeastern Atlantic where the highest occurred in the middle of Biscay Bay (12.67×108 atoms/L) and all the 129I data here are higher than 4.0×107 atoms/L. Five pronounced peaks of 129 I (>2×108 atoms/L) are observed along the surface water transect. However, if these distinct peaks are not taken into consideration, average concentration of 129I in the sampled transect is less than 1×108 atoms/L (mean 6.9×107 atoms/L) and also does not vary a lot in the open sea surface water. Despite nearly similar behavior for 129I- and 129IO3- in the sampled surface water, the concentrations of 129I- show a relatively wider range (0.07-5.73×108 atoms/L). Additionally, 129IO3- concentrations are normally higher than that of 129 I in the sampled region with a few exceptions. The isotopic ratio of 129 127 I/ I has the same trend as that of 129I, which varies between 1.82×10-10 and 5.69×10-9 with an average value of 6.63×10-10. The same pattern also appears for the ratio of 129I-/127I- and 129IO3-/127IO3- and the atomic ratios of 129 127 I/ I for iodide is significantly higher than those for iodate in the investigated region. Figure 4.7: Iodine isotopes (127I and 129I) and their species in the Atlantic seawaters. (a) Variations of isotopic ratio of 129I/127I, 127I-/129I- and 127IO3-/129IO3- in the sampled transect along the northeastern Atlantic Ocean. (b) Concentration of 129I and its species (129I- and 129IO3-) along the sampled transect in the northeastern Atlantic Ocean. The error bars show the analytical uncertainty of one sigma. Higher values of 129I-/129IO3- compared with 127I-/127IO3- are found in all seawater samples (Figure 4.8). Some 129I-/129IO3- values, however, are close to the corresponding 127I-/127IO3- levels which are interpreted to reflect a local effect. This may either be related to older water ventilated to the surface during winter, or the small-scale accelerated redox cycle induced by local 41 chemical or biological characters in these waters. The 129I-/129IO3- value varies from 0.14 to 2.02 in samples where the highest level is located in the region between Madeira and the African coast. Most of the ratio for 129I lies at 0.3-0.7 while the 127I-/127IO3- ratio is normally below 0.2. High values of 129 - 129 I / IO3- (above 0.7) are thought to be directly linked to the large-scale circulation pattern as described in the next section. Similar to 129I and 129 127 I/ I, there are five peaks observed for 129I-/129IO3- which shows decreasing values towards the north. Unlike the 129I-/129IO3-, the ratios of iodide to iodate for 127I remain nearly constant in the sampled surface water transect. On average the value for 127I-/127IO3- is 0.14, which is a factor of five lower than that of 129I-/129IO3- (0.65). Figure 4.8: Variations of 129I-/129IO3- and 127I-/127IO3- along the sampled transect of the northeastern Atlantic Ocean. Colored zones represent 129I species in samples that is close to (< 0.3, pink), approaching (0.3-0.7, green) and far from reaching (> 0.7, yellow) the iodine redox equilibrium in seawaters. Concentrations of 129I show about 30 times difference in surface seawater and with an average of 1.53×108 atoms/L in the sampled transect in 2010. In addition, the ratio of 129I/127I also shows a 30 times variation between the low and high values (1.82-56.85×10-10 atoms/atoms). Both of these values are 2-4 orders of magnitude higher than the natural 129I levels in the ocean (estimated at ~105 atoms/L for 129I and ~10-12 for 129I/127I), which implies strong influence of anthropogenic nuclear activities along the European Coasts. Accordingly, our data suggest that, even the lowest 129I value measured (4.3×107 atoms/L and 1.82×10-10 atoms/atoms for 129I/127I ratio) is higher 42 than the background level of the nuclear weapon testing. This again indicates that the surface water of the northeastern Atlantic Ocean had been labeled by a nuclear fuel reprocessing signal, either from direct marine discharge or from atmospheric 129I releases. In general, iodate is the dominant species of dissolved iodine for both 127I and 129I in the surface waters of the sampled northeastern Atlantic. More than 75% of 127I-iodate is observed in these surface waters, which is comparable to seawaters collected in any other ocean regions (Tsunogai and Henmi, 1971; Truesdale, 1978; Elderfield and Truesdale, 1980; Jickells et al., 1988; Wong, 1995; Campos et al., 1996; Wong and Cheng, 1998; Campos et al., 1999; Farrenkopf and Luther Iii, 2002; Waite et al., 2006; Bluhm et al., 2011). However, for the species of 129 I, there is evidently a higher concentration of iodide compared to that of 127 I. This huge discrepancy of iodine species for 127I and 129I probably reflects different sources of iodine isotopes. 4.3 Sources of 129I 4.3.1 Sources of 129I- in the North Sea Considerable variation of iodide/iodate values for 129I in the North Sea was observed compared with that for 127I. This feature implies a reduction of iodate in the North Sea as the English Channel water parcels moves northward. Despite the positive correlation between 127I and salinity, the insignificant correlation between iodide and salinity indicates that part of the 129IO3is locally reduced. All these features provide evidence for a reductive environment within the North Sea, possibly mediated by bio-activities. An earlier study (Hou et al., 2007) reported a rapid reduction of 129IO3- in the continental European coastal areas and the German Bight. 129I-/129IO3values as high as 50 were observed in the estuary of Elbe River and they related this feature to the combination of chemical and biological processes. Our samples were generally located in the open North Sea, distant from the coastline. Therefore, a significant part of 129I- found in our northern samples might be associated with diffusion and transportation of 129I--rich water that originated from coastal areas, particularly from the hypoxic German Bight. The differences between 129I/127I isotopic ratios for iodide and iodate are significantly larger in the southern samples than those in the north. This feature indicates that the major source of 129I- in northern samples was water masses bringing other than local production. The west Wadden Sea is also a shallow area with estuarine properties characterized by relatively low salinity and large loading of organic matter. Additionally, very low oxygen saturation on its tidal mudflat was reported and seasonal episodic oxygen deficiency occurred in its interior basin (Van 43 der Veer and Bergman, 1986; Hoppema, 1991). Thus the western Wadden Sea should be another potential source of newly produced 129I-iodide under anoxic conditions and further investigation of 129I within this region is needed. 4.3.2 Sources of 129I in the northeastern Atlantic Ocean For the 129I inventory, the influence of direct marine discharges and later transport by ocean current systems play a major role for the concentration in the surface water of the investigated area. The 129I concentration and 129I/127I value in samples north of Cape Finisterre (Spain) analyzed here also confirm the influence from north, where a gradually decreasing pattern is documented southward. Therefore, the 129I level in the north part of the investigated region exhibited a combined contribution from Sellafield and La Hague that could be further advected southward. Seawaters with high 129I concentration south of 40°N clearly imply a different source other than the influence of Sellafield and La Hague from the north. These three isolated peaks do not seem linked to waters from the north due to the general pattern of meridional water movement with respect to the Portugal Current system in this region (Fiúza et al., 1998). This is also confirmed by speciation analysis of 129I that shows in the southern three peaks, iodide as the main species in the surface water column. In addition to the two NRFs mentioned above, the Marcoule NRF (France), which is located on the banks of Rhone river, directly released 45 kg of liquid 129I to the river and 145 kg of 129I to the atmosphere during its operation until 1997 (Hou et al., 2009b). Considering 50% of atmospheric 129 I deposited in the vicinity of the plant and assume all 129I has eventually injected into the Mediterranean Sea through continent runoff, about 120 kg of 129I was calculated in the Mediterranean Sea. As a result, the average concentration of 129I would be about 1.5×108 atoms/L if the 129I is homogeneously mixed in the Mediterranean water. Considering the large amount of 129I released by Marcoule reprocessing plant and the relatively long residence time of iodine (~1800 y) compared with the water turnover time (~1000 y) in the Mediterranean Sea, the Mediterranean water could be a source of 129I to the North Atlantic Ocean. Seawater and algae samples taken from the Mediterranean Sea show a comparable 129I level with our samples that show high 129 I level in the south, suggesting a Mediterranean origin of these waters (Osterc and Stibilj, 2008; Pham et al., 2010). To our best knowledge, there is no 129I speciation in the Mediterranean Sea. However, 129I-/129IO3- values in the English Channel suggest that the source water of 129I-/129IO3- from nuclear reprocessing facilities should contain high values (Hou et al., 2007) and it is reasonable to conclude a high 129I-/129IO3- in the Mediterranean water. Moreover, high iodide concentration is normally found in coasts, bays, estuaries and semi-enclosed basin (Wong, 1995; Tian et al., 1996; Hou et al., 44 2007). Therefore, seawater samples south of 40°N that are characterized by high 129I and 129I-/129IO3- may represent direct influence from the Mediterranean overflow plume that was captured during the cruise. Figure 4.9: Concentrations of 129I (108 atoms/L) along the transect and suggested surface 129I pathways from the English Channel and the Strait of Gibraltar (dashed line). Red regions represent major coastal upwelling and solid blue lines show the spreading pathways of deep Mediterranean water. The nuclear reprocessing facilities (NRFs) are marked as stars. We suggest that the surface waters at 36.5°N may reflect the propagation of a branch of this onshore water westwards towards the open ocean after leaving Cape St. Vicente when easterlies dominated (Figure 4.9). Moreover, a poleward surface current has been reported off the west coast of Portugal during winter when the wind is blowing northward (Frouin et al., 1990). When this water reaches the Cape Espichel, local coastal morphology, shelf/slope bathymetry as well as propagation of eddies make the 129I plume extend seaward. The other two high-129I samples, which occur in the middle 45 of Madeira and African coast, however, are related to the onshore flow of the Canary Current, which with its one branch separated from the northern Morocco coast (Johnson and Stevens, 2000), may transport 129I from the Strait of Gibraltar to further west around 14°W. 4.4 129I inventories in the North Sea and the English Channel Here we made a simple calculation of 129I inventory based on data from 2005 (Michel et al., 2012) and applying the release function from the NRFs (Figure 1.2). The entire North Sea is divided into three compartments and for the relatively deep regions (i.e. the Norwegian Trench and the central and northern part of the North Sea), the compartments are subdivided into two layers separated by mean thermocline depth of 30 m. Distribution of 129I inventory in each 10×10 km water column is shown in Figure 4.10. The results show that about 114 kg 129I resided in the North Sea water. Among this, more than 90% of 129I inventory resides in the German Bight and the southern North Sea. Figure 4.10: Distribution of gridded 129I inventory (in 1022 atoms) in the North Sea in 2005. 129 I inventories in the central and northern portion of the North Sea were more than two times higher than that in the Norwegian Trench and >90% was accumulated in the upper layer whereas in the Norwegian Trench, the 46 129 I inventory is comparable in surface and deep layers. However, inventories of gaseous (atmospheric) 129I release only account for 5% and 4% of the liquid (marine) discharges from Sellafield and La Hague until 2005, respectively. These percentages were even decreased to < 3% and < 1% in the recent five years, respectively. Therefore, atmospheric release of 129I is relatively insignificant compared to the marine discharge. Accordingly, the estimated 129I inventory in the North Sea accounts for about 3.4% of the individual La Hague marine discharges, and 2.4% of the combined marine discharges from La Hague and Sellafield until 2005. During 2006-2010, the 129I marine discharges from La Hague were 956 kg, which will add about 32.5 kg of 129I to the inventory in the North Sea until 2010 based on the estimated 3.4% mentioned above. Thus if we simply ignored the 129I contribution from Sellafield and the loss of 129I to the Baltic Sea in 2010, 129I inventory in the North Sea would be 147 kg until 2010. For inventory calculation of the English Channel, gridding with 10 km×10 km and subdivision into western and eastern parts was applied. The relatively deep western English Channel is further subdivided into two layers separated by mean thermocline depth of 20 m. The estimated inventory of 129 I in the English Channel is 78 ± 12 kg, in which over 90% resides in the east of La Hague (Figure 4.11). The inventory in the western English Channel, however, is much less. Even when the well mixed water column in the whole western part is considered, the inventory in the west English Channel is still only one-fifth of that in the east. Additionally, though the volume of the surface layer is nearly three times less than that in the bottom, it accounts for about 84% of the total inventory of the western part. Apart from total 129I, a first inventory estimation of 129I species in the English Channel is also calculated. Despite the high 129I-/129IO3- that occasionally occurred in some seawater samples, most of the inventory is iodate-dominated in the English Channel. Furthermore, distribution and proportion of 129I- and 129IO3- in each compartment generally follow the same pattern as total 129I. This distribution behavior of 129I and its species agrees well with the major water movement within the channel. Direct marine release from La Hague plays a major role in terms of 129I inventory in the English Channel and the estimated 129I in 2010 constitutes 1.77% of liquid release by La Hague since the start of its operation until 2010. Compared to neighboring areas, the inventory of 129I in the English Channel is nearly 4 times higher than that in the Baltic Proper (Yi et al., 2010), but about half of the estimated inventory in the North Sea. 47 Figure 4.11: Inventories of 129I (in 1020 atoms) in each gridded cell in the English Channel in 2010. In general, there is a total of 255 kg of 129I in the English Channel, North Sea and the Baltic Sea, which constitutes only 5.8% of marine discharges from La Hague. This feature implies that most of 129I released from La Hague has left the North Sea and partly resides in the sediments. 4.5 Transformation among iodine species in seawater In marine waters, iodine mainly exists as iodate, iodide and a minor fraction dissolved organic iodine (DOI). In the state of thermodynamic equilibrium, iodate should be the only detectable form of iodine in oxygenated seawater. However, significant iodide is often observed in the ocean surface layer. In some coastal bays, or anoxic basins such as the Baltic Sea and the Black Sea, 48 iodide could be the major form (Ullman et al., 1988; Luther Iii and Campbell, 1991; Luther Iii et al., 1991; Truesdale et al., 2001). Concentration of iodine species varies a lot with respect to depth and locations in the sea. Although the conversion between iodine species in natural water is not well understood, it might be attributed to chemical, biological and photochemical processes. Several abiotic mechanisms have been suggested to reduce iodate to iodide under anoxic conditions, with the presence of reducing agents such as iron, manganese and sulfur species (e.g. thiols, bisulphides, etc.) (Jia-Zhong and Whitfield, 1986; Anschutz et al., 2000): 1/2 Mn2+ + 1/6 IO3- + 1/2 H2O ↔ 1/2 MnO2 + 1/6 I- + H+ Photochemical reduction of iodate may also be important which was supported by laboratory experiments (Spokes and Liss, 1996) and this process seems strongly catalyzed by dissolved organic matter: hv IO3- + 6H+ + 6e- → I + 3H2O The presence of iodide in the oxic euphotic zone is suggested by reduction of iodate mediated biologically. These biotic processes include bacterial reduction with the enzyme nitrate reductase (Tsunogai and Sase, 1969; Wong and Hung, 2001), and the phytoplankton activities (Moisan et al., 1994; Tian et al., 1996; Wong et al., 2002). The reduction of iodate to iodide does not seem to be related to concentration of primary production but more linked to cell senescence (Tian et al., 1996; Bluhm et al., 2010). In addition, iodide may be released from sediment at the sediment/seawater interface where remineralization of organic materials is intensified (Ullman and Aller, 1980; Aldahan et al., 2007): C-H-O-N-I + SO42- → CH4 + CO + H2S + NOx + 2IConversely, some experiments by laboratory cultures argued that neither the photochemical process nor organic activity were a substantial effect on iodate reduction. In these experiments, little change of iodine species was reported under phytoplankton bloom or high light intensity conditions (Brandão et al., 1994; Wong et al., 2002; Truesdale et al., 2003). Thus a hypothesis was proposed that iodate may be used to mop up electrons which present in excess of photosynthetic needs (Waite and Truesdale, 2003). When the iodide is produced, then oxidation into iodate is sluggish, because the redox of iodine in marine waters is suggested to be a slow process. The mechanisms that govern the iodide oxidation are still unclear. Photochemical oxidation may occur with the help of strong UV radiation or oxidants (e.g. H2O2, O3, etc.) (Wong and Zhang, 2008): 49 hv I2 + 2OH2I- + 1/2 O2 + H2O → I + H2O2 → HOI + OH- The reactive transients of I2 and HOI tend to return to I- or incorporated into organic materials rather than the further oxidation of HOI to iodate. Therefore, the transformation of iodide to iodate in seawater might also be biologically mediated (Luther George et al., 1995). The significant quantities of dissolved organic iodine (DOI) in some coastal and estuarine waters may add a new loop of iodine geochemistry in seawaters (Wong and Cheng, 2001). DOI can be formed from iodate and iodide with reactive oxidants, or released from decomposition of organic matter. Conversely, decomposition of DOI can introduce iodide to the water column and this process was suggested to be photochemical or biological (bacterial mediated) (Luther Iii et al., 1991; Luther George et al., 1995; Wong and Cheng, 2001b). In most of our seawater samples along the transect, although iodate confirmed to be predominantly occurring, significant iodide can also be observed, particularly in the North Sea and the English Channel. This is in accord with previous reports and it is likely influenced by biotic processes. However, since the redox of iodine is suggested to be a slow process, it seems that relatively large variations of iodine species composition in some regions, such as the North Sea and the southwestern English Sea, mainly reflect water mass mixing and migration rather than interconversions between iodine species. For 129I, in most cases high 129I-/129IO3- were found, especially in the eastern North Sea, compared to 127I-/127IO3-. This behavior is attributed to propagation of inshore waters that bear a high concentration of 129 I into the German Bight, where strong iodate reduction happens. High 129Iin some Atlantic water samples may also occur through reduction in the coastal areas of the northwest Mediterranean Sea, which might be driven by mixed mechanisms mentioned in this section. In addition, DOI may play an insignificant role since the values of (129I- + 129IO3-) / 129I in all samples range between 0.90 and 1.10, which lie within the analytical error range. Moreover, in open seas, DOI content is normally low and this was confirmed by seawater samples taken from the North Sea (Hou et al., 2013). 50 5. Environmental impact of 129I At present, 129I is not regarded to pose a significant human health risk. However, because of its high mobility and long half live, as well as the fact that more than 90% of spent nuclear fuel is still waiting for reprocessing, concentrations of anthropogenic 129I are expected to increase in the studied regions. Due to its low beta-decay energy, 129I may not constitute a significant external exposure hazard. Soils from Sweden indicated about 1.0 Bq/m2 for 129I, which was much less radioactivity than that from 137Cs (103-105 Bq/ m2) (Aldahan et al., 2006). However, elevated levels of 129I in water, atmosphere and soil will finally find its way into the food chain, which can result in internal exposure effects. Iodine is generally concentrated in organisms which are associated with the human diet. Osterc et al. (Osterc and Stibilj, 2012) investigated 129I concentrations in mussel tissues collected in the Mediterranean Sea and their results showed a high 129I/127I values (10-9-10-8). A wider ratio range (10-1110-8) was observed in milk from central USA (Oliver et al., 1982). It is known that seaweed can concentrate iodine to extremely high levels, and the 129 127 I/ I is normally higher than 10-8 in seaweeds sampled from western and northern European coast (Hou et al., 2000a; Yiou et al., 2002; Keogh et al., 2007). The bioavailability of iodine depends on its species in the environment. Water soluble and exchangeable fractions of 129I in soil are preferentially taken up by plants (Hou et al., 2009b). For mammals and human beings, KI is more easily assimilated than iodine bonded to organic macromolecules (Hou et al., 2009b). Although most of iodine uptake is relatively quickly excreted out of human body (4-5 months), the transformation path is commonly through the mammal thyroid (Hou et al., 2009b; Jahreis et al., 2001). 129I/127I in animal (or human) thyroid present a low level of ~10-10-10-9 in areas far from known-129I sources, such as NRFs and the Chernobyl region (Chao and Tseng, 1996; Handl, 1996; Hou et al., 2000b). Accordingly, much lower values of 129I/127I that are close to pre-anthropogenic level were reported from Argentina (Negri et al., 2012). However, in Chernobyl fallout regions or vicinity areas of NRFs, the 129I/127I ratio dramatically increased to 10-8-10-5 (VanMiddlesworth and Handl, 1997; Fréchou et al., 2002). Considering about 10 mg of iodine in human thyroid, the highest 129I/127I ratio of ~10-5 (0.65 Bq of 129I) results in equilibrium annual dose equivalent to the thyroid of ~ 10-2 mSv/y (Soldat et al., 1973). This is about two orders of magnitude lower than annual maximum effective dose for an adult which is 51 the suggested permissible level of European Nuclear Society (1mSv/y) (http://www.euronuclear.org/info/encyclopedia/d/doselimitvalue.htm). The 129I in table salts that are used in Sweden varies at 1-4×10-6 Bq/kg (Aldahan et al., 2006), which is equivalent to 7.3-29.2×10-7 Bq/y if we assume 2 g of daily uptake of salt, thus the radioactivity from daily table salt can be neglected. Similarly, insignificant radiation of 129I through inhalation is estimated (10-7-10-6 Bq/y) if the average 129I concentration of 104-105 atoms/m3 in atmosphere is applied (Santos et al., 2005). However, it is radioactive hazard of water due to large amount of daily water consumption possible (~2L/day for adult). Aldahan et al. (Aldahan et al., 2006) reported that 129 I in Swedish tap water is ~108 atoms/L. Therefore, daily intake of 129I through drinking water is 2×108 atoms and the corresponding radioactivity is 200 times higher than that from table salt and inhalation. These features suggest an insignificant radiation hazardous presently contributed by 129I intake. Concentration of 129I in seawaters collected in the northeastern Atlantic Ocean was slightly lower than in the tap water in Sweden, while the water samples taken from the North Sea and the English Channel showed a much higher 129I level (1×10-5 – 6×10-4 Bq/L). However, the external radiation of 129 I is less harmful because 129I is a soft beta-emitter. Thus, the potential radiological risk for human beings in the western European coast and the Baltic regions is accompanied with internal dose, when humans consume seafood which concentrates surrounding 129I to an extremely high level. Fish otolith has been used as an environmental marker for fish lifetime and migration pathway (Campana, 1999). The majority of elements deposited in otolith originate from water, with a minor fraction coming from food sources. 127I was already found in otolith and since 129I has been detected in fish tissues and flesh (reference IAEA), 129I may also be incorporated into the otolith (Gemperline et al., 2002; Pham et al., 2006). Thus, variations of iodine isotopes (129I and 127I) and their species in seawater are expected to be reflected in the growth of otoliths (e.g. Atlantic cod and Atlantic herring). This possibility can open a new interesting approach where growth and migration pathways of fish can be traced using the ratio of 129I/127I. 52 6. Conclusions This thesis concerns the distribution and sources of iodine isotopes (127I and 129 I) and their species (iodide and iodate) in ocean waters collected from the North Sea, the English Channel, the Celtic Sea and the northeastern Atlantic Ocean. Additionally, a review of 129I distribution in the oceans was performed. The main conclusions and findings are summarized below: 1. Concentrations of 129I in the world oceans range several orders of magnitude, but were all at least 130 times higher than the pre-nuclear era level. The highest values were restricted to the English Channel and the Irish Sea, whereas low levels occurred in regions that are far from major NRFs. Currently, most available oceanic 129I data come from areas north of 60°N in the Atlantic Ocean, Nordic Seas and the Arctic Ocean, while in most parts of the Pacific, Indian and Southern Oceans, data are insufficient which hinder tracking global oceanic distribution of 129I. Moreover, although it would be an additional powerful tool in iodine geochemistry, few 129I data on speciation of 129 I was available for the world oceans. 2. A more than one order of magnitude difference in 129I concentrations was observed in the English Channel water between west and east of La Hague peninsula. This observation suggests strong impact of water movement towards the east and into the North Sea. The results show that 129I in the southern Celtic Sea is mainly originated from La Hague, although some clues implied influences from Sellafield. 3. In general, changes in the 127I and 127I-/127IO3- are comparable to data from other marine waters which are related to natural distribution patterns. In addition, occurrence of deepwater upwelling in the western English Channel was detected through enhanced 127I concentration in surface water. 4. 129 I inventories in the North Sea and the English Channel were estimated as 147 kg and 78 kg until 2010, respectively. Of these, more than 90% of 129I resides in the eastern English Channel, the southern North Sea and the German Bight. Total inventory of 129I in the Eng53 lish Channel, the North Sea and the Baltic Sea is about 255 kg, which constitutes only 5.8% of marine discharges from La Hague. 5. Iodate is the dominant iodine species for both 127I and 129I in most seawater samples from the North Sea to the Atlantic Ocean. Besides, iodide to iodate ratio for 129I was normally higher than that of 127I, which reflects different sources of the two isotopes. Temporal variation of 129I and its species in the North Sea show an off-shore propagation and dilution of coastal water, which can be coupled with interannual climate oscillation over this region. 6. The average value of 129I-/(129IO3- + 129I-) in source water (English Channel) shows little temporal variation which was crucial for estimation of iodine species interconversion. Use of this constant ratio resulted in identifying water transport from the western English Channel via Biscay Bay as the main source of 129I in the northeastern Atlantic Ocean. 7. Speciation analysis of 129I suggests a slow process of 129I- oxidation in the open sea, where about 9% of 129I- is annually oxidized. This feature means that it takes at least 10 years for the 129I-/129IO3- pair to reach their natural equilibrium as the water is transported from the English Channel. 8. The results of 129I and its species suggest that the Mediterranean Sea is another source contributing to the inventory of 129I in the North Atlantic Ocean. Therefore, our research proposes a potential prospective of labeling MOW (Mediterranean Outflow Water) using 129I speciation. Depth profiles of 129I in the Gibraltar Strait and the Gulf of Cadiz are needed for further investigations. 9. The environmental impact of 129I can be a multi-axis tool, one is related to radioactivity hazards which is presently seems not harmful. The other axis is a potential tracer of water masses exchange and circulation. The third axis is a tool for ecosystem variability, where migration of fish and other marine species can be traced. 54 7. Acknowledgements There are many people whom I wish to thank during my life in Uppsala. First I would like to thank my supervisor Ala Aldahan, who has offered me invaluable support during my academic studies as a PhD student. I have profited greatly from his expert guidance and encouragement throughout these years. Thank you for teaching me a lot about research and how to express ideas in a structured and scientific way. The completion of this thesis would not have been possible without his help. I would also like to extend my sincere thanks to my co-supervisor Göran Possnert, for your kind help with AMS analysis, as well as the patience and useful suggestions in the course of writing papers. I have benefited so much from good discussions with you, and impressed a lot with your good sense of humor. I would like to express my gratitude to my co-supervisor Rickard Pettersson, who patiently guided me with the application of ArcGIS step by step. Also thanks for your great course of Geoinformatics. I gratefully acknowledge Xiaolin Hou for his support during these years. I appreciate his great scientific attitude and thanks for the excellent advice about research. Thank you for your help with chemical experiment in Denmark and I have learnt a lot from you in both laboratory work and scientific writing. Gratitude also goes to my friends Keliang Shi, Jixin Qiao, Haijun Dang and Violeta Hansen, who helped me a lot and shared the wonderful time in Denmark. Special thanks to my dear friends Peng Yi and Biao Wang, for your close friendship and considerable help. You have always been there when I needed you and our real friendship never ends. I would like to thank Inger Påhlsson and Ann-Marie Berggren for your assistance during my laboratory work in Uppsala. Additional gratitude extends to Ann-Marie Berggren for your constructive comments on my thesis. Warm thanks go to all my present and former PhD students and colleagues in LUVA, including Diana, Lebing, Agnes, Zhibing, Estuardo, Liang, Anna, Elias, Carmen, Maria, Kristina, Beatriz, Martin, Fritjof, Lichuan, Sergey, Byeongju, Wasana and so many more. I would like to thank all of you for the friendly environment created at the department. Thanks to Chongyu Xu, Hemin Koyi, and Kevin Bishop who helped and encouraged me in the department. 55 I would like to thank my Chinese friends and colleagues within and outside the department, for helping me to adapt the life in Uppsala and make the days interesting and fun: Fengjiao Zhang, Juntian Zheng, Jianliang Wang, Chunling Shan, Dixiao Bao, Ping Yan, Yue Hong, Haizhou Wang, Can Yang, Jiaqi Li, Taigang Zhou, Xu Quan, Zhina Liu, Zhihong Zhao, Fuguo Tong, Zhifei Zhang, Yan Wang, Ting Zhang, Fei Huang, Hongling Deng, Tang Xu, Chi Zhang, Lei Zhang. Thanks to Tomas Nord for excellent help dealing with computer problems. Thanks to Leif Nyberg, Anna Callerholm and Taher Mazloomian for their help during office hours. I gratefully acknowledge Susanne, Eva, Fatima and the administrative stuff who made life easier at the department. I would like to thank Lars Andersson, Bodil Thorstensson and all members on the research vessel Argos from the Swedish Meteorological and Hydrological Institute (SMHI), for their help during the one-week sampling campaign in the Baltic Sea. Thanks to Anna Sturevik Storm, who shared a nice time with me during the Antarctica expedition. Thanks to Kenneth Nilsson for the interesting story about the Arctic cruise and I like the good sense of humor. I am also deeply indebted to Stefan Karlsson, Erik Andersson and all the crew members on icebreaker Oden. Thanks for their help which made the Antarctica expedition a great memory for life. Thanks to Henrik Kylin for kindly provide us with some of the electrical transformers needed during the expedition. The Antarctica expedition was funded by the Swedish Polar Research Secretary and the US National Sciences Foundation (NSF). Special thank to the financial support from the China Scholarship Council (CSC). The analysis of iodine isotopes was funded by the Tandem Laboratory, Uppsala University, and the Center for Nuclear Technologies, Technical University of Denmark. I would like to thank my former supervisor in China, Ruyun Wang, for his encouragement and giving me the opportunity to study in Sweden. Last but not least, I wish to thank my girlfriend Sha Wei, and my beloved family for their continuous support and encouragement. I will try my best to make you proud of me. 56 8. Summary in Swedish 129 I är en radioaktiv jodisotop med lång halveringstid som sedan 1940-talet tillförts miljön i stora mängder som en följd av antropogena verksamheter. Eventuella risker till följd av isotopens radioaktivitet är inte undersökta i detalj. 129I har däremot framgångsrikt använts som spårämne i marina system för att undersöka havsströmmar, utbyte av vattenmassor, vattnets transportvägar och allmän jodgeokemi. Undersökningar av hur isotopen sprids i vatten har mestadels fokuserat på Nordsjön, de nordiska haven, Östersjön och Ishavet eftersom de utgör potentiella transportvägar för 129I-utsläpp från de två stora kärnbränsleupparbetningars anläggningar i Engelska kanalen (La Hague, Frankrike) och Irländska Sjön (Sellafield, Storbritannien). Kunskaperna om 129I i andra marina miljöer är dock fortfarande bristfälliga. I denna avhandling presenteras nya data om spridningsmönster och källor för 129I i Atlanten genom analyser av ytvatten längst en transekt från Nordsjön, genom Engelska kanalen, vidare in i Keltiska havet och genom nordöstra Atlantiska oceanen. Ytvattensprovtagningen genomfördes under oktober och november 2010, som en del av Svenska Polarforskningssekretariatet och amerikanska National Science Foundations (NSF) Antarktis expedition. Dessa prover analyserades både vad avser totala mängder av två jodisotoper (127I och 129I) samt deras olika kemiska former (jodat och jodid), med hjälp av en omfattande kemisk separation, följt av mätningar med accelerator masspektrometri (AMS) och induktivt kopplad plasma masspektrometri (ICP-MS). Avhandlingen innehåller en sammanfattning av data om 129I i världshaven (Paper I), där det framgår att 129I koncentrationer i ytvatten från Atlanten, Stilla havet och Indiska oceanen är minst 1-3 magnituder lägre än i Ishavet. Resultaten av provtagningarna på jodisotoper (127I och 129I) och deras kemiska former längs transekten presenterade i fyra artiklar (Papers II-V). Av dessa analyser framgår att 129I-halten i havsvatten längs transekten uppvisar en betydande förändring med en ökning på cirka 40 gånger från Nordsjön till Engelska kanalen, där det högsta värdet påträffades nära Doverkanalen. Denna höga 129I-halt följs av en dramatisk minskning på mer än fyra magnituder från Cap de La Hague till det öppna havet. Alla 129I-koncentrationer och 129I/127I-kvoter i de insamlade ytvattensproverna låg över nivåerna för det globala nedfallet från kärnvapenprov. Förutom analyser av 129I-koncentrationer och 129I/127I-kvoter, visar resultaten av jodisotopernas olika kemiska former på en ny möjlighet att förfina 57 användande av 129I som spårämne i marina miljöer. Fram till idag har det enbart funnits data som visar på bildande av de olika formerna av 129I i Nordsjön och Östersjön men i denna avhandling utökas denna viktiga information till andra havsområden. Tydliga geografiska källorområden för dessa två jodisotoper framträder av den stora skillnaden mellan jodid/jodat för båda 127I och 129I. Dessa karaktärsdrag antyder att den naturliga termodynamiska jämvikten mellan isotoperna inte föreligger i haven och att oxidationsprocessen där 129I omvandlas till 129IO3- är långsam i det öppna havet. Förändringen av 129I-/129IO3- i havsvatten kan således användas för att identifiera 129I-källor vilket i sin tur leder till slutsatsen att den huvudsakliga källan till 129I i nordöstra Atlanten kan hänföras till Keltiska havet och inte Labradorhavet. Skillnader i 129I-/129IO3- kvoten i proverna från Keltiska sjön tyder på en påverkan från utsläppen från Sellafields kärnbränsleupparbetning. Utsläppen från La Hague å andra sidan förefaller främst bidra till 129I i västra Engelska kanalen. Denna förekomst tillsammans med ett möjligt södergående vattenflöde från Irländska sjön, har medfört att Keltiska sjön kan ses som ett 129I-förorenat område. Om en 129I-plym förflyttar sig ytterligare, vid gynnsamma vindförhållanden, kan den även komma att påverka nordöstra Atlanten och då särskilt Biscayabukten. Höga 129I-/129IO3- halter i havsvatten från södra Nordsjön visar på en icke kustnära spridning av europeiska kustvatten, vilket kan vara kopplat till extrema klimatvariationer. De observerade 129I/129I värdena från Engelska kanalen ger en tydlig bild av de troliga ingångsvärdena till La Hagues avloppsledningar och proportionerna visar på små temporala och rumsliga förändringar inom kanalen. Fem vattenprover från nordöstra Atlanten karakteriserades av höga 129I-koncentrationer och skillnader i 129I-/129IO3-halten att dessa vattenmassor kan förklaras med att olika källorområden föreligger, där de norra proverna associerades med utsläpp från Sellafield och La Hague medan de övriga proverna antyder Medelhavsvatten som källa till 129I. Förslagsvis kan 129I och dess kemiska former (129I- och 129IO3-) användas för att undersöka spridningen av Medelhavsvattnets utflöde, Mediterranean Outflow Water (MOW). Potentiella tillämpningar av 129I/127I i marina ekosystem diskuteras även i avhandlingen. Slutligen görs uppskattningar av totala mängden 129I i Nordsjön och Engelska kanalen där resultaten tyder på att > 90 % av 129I förekomer i östra Engelska kanalen och södra bukten. Största delen av de 129I-utsläppen som kommer från La Hague och Sellafield har dock borttransporterat med konvektivt flöde från Engelska kanalen-Nordsjön-systemet och kan därigenom nå Atlantens subtropiska havsströmsområde. För närvarande anses inte 129I skadligt påverka människors hälsa, men på grund av dess höga mobilitet och långa halveringstid, liksom det faktum att mer än 90 % av allt använt kärnbränsle fortfarande väntar på upparbetning kan en framtida potentiell risk föreligga. Fiskotolit, som uppvisar årsringar och därmed visar på fiskens livslängd och hur den migrerar, har används som ett intressant miljöarkiv med möjlighet att även återspegla jod och dess isotoper efter58 som både 127I och 129I har påträffats i denna vävnad. Därmed kan kunskaper om jodisotoperna 129I och 127I och deras olika kemiska former i havsvatten öppna för en ny intressant metod där tillväxt och migrationsvägar för fiskar kan studeras. 59 9. References Aldahan, A., Alfimov, V., and Possnert, G. (2007a). 129I anthropogenic budget: Major sources and sinks. Applied Geochemistry 22, 606-618. Aldahan, A., Englund, E., Possnert, G., Cato, I., and Hou, X. (2007b). Iodine-129 enrichment in sediment of the Baltic Sea. Applied Geochemistry 22, 637-647. Aldahan, A., Kekli, A., and Possnert, G. (2006). Distribution and sources of 129I in rivers of the Baltic region. Journal of environmental radioactivity 88, 49-73. Aldahan, A., Persson, S., Possnert, G., and Hou, X. L. (2009). Distribution of 127I and 129I in precipitation at high European latitudes. Geophys Res Lett 36, L11805. Alfimov, V. (2005). Accelerator Mass Spectrometry of 36Cl and 129I. Analytical Aspects and Applications, Uppsala, Dissertation. Alfimov, V., Aldahan, A., and Possnert, G. (2004a). Tracing water masses with 129I in the western Nordic Seas in early spring 2002. Geophys Res Lett 31, L19305. Alfimov, V., Aldahan, A., Possnert, G., Kekli, A., and Meili, M. (2004b). Concentrations of 129I along a transect from the North Atlantic to the Baltic Sea. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 223, 446-450. Alfimov, V., Aldahan, A., Possnert, G., and Winsor, P. (2004c). Anthropogenic iodine-129 in seawater along a transect from the Norwegian coastal current to the North Pole. Marine pollution bulletin 49, 1097-1104. Ambar, I. (1983). A shallow core of Mediterranean water off western Portugal. Deep Sea Research Part A Oceanographic Research Papers 30, 677-680. Anschutz, P., Sundby, B., Lefrançois, L., Luther Iii, G. W., and Mucci, A. (2000). Interactions between metal oxides and species of nitrogen and iodine in bioturbated marine sediments. Geochimica et cosmochimica acta 64, 2751-2763. AREVA Cumulative release results report. France http://www.areva.com/EN/operations-2315/cumulative-release-results-reportfor-the-areva-la-hague-plant.html. Atarashi-Andoh, M., Schnabel, C., Cook, G., MacKenzie, A. B., Dougans, A., Ellam, R. M., Freeman, S., Maden, C., Olive, V., Synal, H. A., and Xu, S. (2007). 129I/127I ratios in surface waters of the English Lake District. Applied Geochemistry 22, 628-636. Bachhuber, H., and Bunzl, K. (1992). Background levels of atmospheric deposition to ground and temporal variation of 129I, 127I, 137Cs and 7Be in a rural area of Germany. Journal of environmental radioactivity 16, 77-89. Bailly du Bois, P., and Guéguéniat, P. (1999). Quantitative assessment of dissolved radiotracers in the English Channel: Sources, average impact of la Hague reprocessing plant and conservative behaviour (1983, 1986, 1988, 1994). Continental Shelf Research 19, 1977-2002. Baker, A. R. (2004). Inorganic iodine speciation in tropical Atlantic aerosol. Geophys Res Lett 31, L23S02. 60 Baker, A. R., Tunnicliffe, C., and Jickells, T. D. (2001). Iodine speciation and deposition fluxes from the marine atmosphere. J Geophys Res 106, 2874328749. Baringer, M. O. N., and Price, J. F. (1999). A review of the physical oceanography of the Mediterranean outflow. Marine Geology 155, 63-82. Barkley, R. A., and Thompson, T. G. (1960). The total Iodine and Iodate-iodine content of sea-water. Deep Sea Research (1953) 7, 24-34. Beasley, T., Cooper, L. W., Grebmeier, J. M., Aagaard, K., Kelley, J. M., and Kilius, L. R. (1998). 237Np/129I atom ratios in the Arctic Ocean: Has 237Np from Western European and Russian fuel reprocessing facilities entered the Arctic Ocean? Journal of environmental radioactivity 39, 255-277. Beasley, T. M., Cooper, L. W., Grebmeier, J. M., Kilius, L. R., and Synal, H. A. (1997). 36Cl and 129I in the Yenisei, Kolyma, and Mackenzie Rivers. Environmental science & technology 31, 1834-1836. Block, C., and Dams, R. (1974). Determination of trace elements in coal by instrumental neutron activation analysis. Analytica Chimica Acta 68, 11-24. Bluhm, K., Croot, P., Wuttig, K., and Lochte, K. (2010). Transformation of iodate to iodide in marine phytoplankton driven by cell senescence. Aquatic Biology 11, 1-15. Bluhm, K., Croot, P. L., Huhn, O., Rohardt, G., and Lochte, K. (2011). Distribution of iodide and iodate in the Atlantic sector of the southern ocean during austral summer. Deep Sea Research Part II: Topical Studies in Oceanography 58, 27332748. Brandão, A. C. M., de Luca Rebello Wagener, A., and Wagener, K. (1994). Model experiments on the diurnal cycling of iodine in seawater. Marine Chemistry 46, 25-31. Broecker, W. S., and Peng, T. H. (1982). Tracers in the sea. Brown, J., Carrillo, L., Fernand, L., Horsburgh, K. J., Hill, A. E., Young, E. F., and Medler, K. J. (2003). Observations of the physical structure and seasonal jet-like circulation of the Celtic Sea and St. George's Channel of the Irish Sea. Continental Shelf Research 23, 533-561. Buraglio, N., Aldahan, A., and Possnert, G. (1999). Distribution and inventory of 129I in the central Arctic Ocean. Geophysical Research Letters 26, 1011-1014. Buraglio, N., Aldahan, A., and Possnert, G. (2001a). 129I in lakes of the Chernobyl fallout region and its environmental implications. Applied radiation and isotopes 55, 715-720. Buraglio, N., Aldahan, A., Possnert, G. Ö., and Vintersved, I. (2001b). 129I from the nuclear reprocessing facilities traced in precipitation and runoff in northern Europe. Environmental science & technology 35, 1579-1586. Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263-297. Campos, M. L. A. M., Farrenkopf, A. M., Jickells, T. D., and Luther Iii, G. W. (1996). A comparison of dissolved iodine cycling at the Bermuda Atlantic Time-series Station and Hawaii Ocean Time-series Station. Deep Sea Research Part II: Topical Studies in Oceanography 43, 455-466. Campos, M. L. A. M., Sanders, R., and Jickells, T. (1999). The dissolved iodate and iodide distribution in the South Atlantic from the Weddell Sea to Brazil. Marine Chemistry 65, 167-175. 61 Carmack, E. C., Aagaard, K., Swift, J. H., MacDonald, R. W., McLaughlin, F. A., Peter Jones, E., Perkin, R. G., Smith, J. N., Ellis, K. M., and Killius, L. R. (1997). Changes in temperature and tracer distributions within the Arctic Ocean: results from the 1994 Arctic Ocean section. Deep Sea Research Part II: Topical Studies in Oceanography 44, 1487-1502. Carpenter, L. J. (2003). Iodine in the Marine Boundary Layer. Chemical Reviews 103, 4953-4962. Chameides, W., and Davis, D. (1980). Iodine: Its possible role in tropospheric photochemistry. Journal of Geophysical Research 85, 7383-7398. Chao, J.-H., and Tseng, C.-L. (1996). 129I concentrations of mammalian thyroids in Taiwan. Science of The Total Environment 193, 111-119. Chatfield, R. B., and Crutzen, P. J. (1990). Are there interactions of iodine and sulfur species in marine air photochemistry? Journal of Geophysical Research 95, 22319-22322,22341. Cochran, J. K., Moran, S. B., Fisher, N. S., Beasley, T. M., and Kelley, J. M. (2000). Sources and transport of anthropogenic radionuclides in the Ob River system, Siberia. Earth and Planetary Science Letters 179, 125-137. Cooper, L., Beasley, T., Zhao, X. L., Soto, C., Vinogradova, K., and Dunton, K. (1998). Iodine-129 and plutonium isotopes in Arctic kelp as historical indicators of transport of nuclear fuel-reprocessing wastes from mid-to-high latitudes in the Atlantic Ocean. Marine Biology 131, 391-399. Cooper, L. W., Hong, G. H., Beasley, T. M., and Grebmeier, J. M. (2001). Iodine129 Concentrations in Marginal Seas of the North Pacific and Pacific-influenced Waters of the Arctic Ocean. Marine pollution bulletin 42, 1347-1356. Dahlgaard, H. (1995b). Transfer of European coastal pollution to the arctic: Radioactive tracers. Marine pollution bulletin 31, 3-7. Duce, R. A., Wasson, J. T., Winchester, J. W., and Burns, F. (1963). Atmospheric iodine, bromine, and chlorine. Journal of Geophysical Research 68, 3943-3947. Edmonds, H., Smith, J., Livingston, H., Kilius, L., and Edmond, J. (1998). 129I in archived seawater samples. Deep-Sea Research Part I 45, 1111-1125. Edmonds, H., Zhou, Z., Raisbeck, G., Yiou, F., Kilius, L., and Edmond, J. (2001). Distribution and behavior of anthropogenic 129I in water masses ventilating the North Atlantic Ocean. Journal of Geophysical Research 106, 6881-6894. Edwards, R. R. (1962). Iodine-129: Its Occurrenice in Nature and Its Utility as a Tracer. Science 137, 851-853. Elderfield, H., and Truesdale, V. W. (1980). On the biophilic nature of iodine in seawater. Earth and Planetary Science Letters 50, 105-114. Englund, E., Aldahan, A., Possnert, G., Haltia-Hovi, E., Hou, X., Renberg, I., and Saarinen, T. (2008). Modeling Fallout of Anthropogenic 129I. Environ Sci Technol 42, 9225-9230. Ernst, T., Szidat, S., Handl, J., Jakob, D., Michel, R., Schnabel, C., Synal, H.-A., Santos Arevalo, F., Benne, I., and Boess, J. (2003). Migration of iodine-129 and iodine-127 in soils. Kerntechnik 68, 155-167. Fabryka-Martin, J., Bentley, H., Elmore, D., and Airey, P. L. (1985). Natural iodine129 as an environmental tracer. Geochimica et cosmochimica acta 49, 337-347. Fabryka-Martin, J., Davis, S. N., and Elmore, D. (1987). Applications of 129I and 36Cl in hydrology. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 29, 361-371. Farrenkopf, A. M., and Luther Iii, G. W. (2002). Iodine chemistry reflects productivity and denitrification in the Arabian Sea: evidence for flux of dissolved species from sediments of western India into the OMZ. Deep Sea Research Part II: Topical Studies in Oceanography 49, 2303-2318. 62 Fehn, U., Moran, J., Snyder, G., and Muramatsu, Y. (2007). The initial 129I/I ratio and the presence of 'old' iodine in continental margins. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 259, 496-502. Fehn, U., and Snyder, G. (2000). 129I in the southern hemisphere: Global redistribution of an anthropogenic isotope. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 172, 366-371. Fehn, U., Snyder, G., and Egeberg, P. (2000). Dating of pore waters with 129I: Relevance for the origin of marine gas hydrates. Science 289, 2332. Fehn, U. H., G. R.; Elmore, D.; Brunelle, T.; Teng, R.; Kubik, P. W. (1986). Determination of natural and anthropogenic 129I in marine sediments. Geophysical Research Letters, Volume 13, Issue 2, p 137-140 Fiúza, A. F. G., Hamann, M., Ambar, I., Dıá z del Rıó , G., González, N., and Cabanas, J. M. (1998). Water masses and their circulation off western Iberia during May 1993. Deep Sea Research Part I: Oceanographic Research Papers 45, 1127-1160. Fréchou, C., Calmet, D., Bertho, X., and Gaudry, A. (2002). 129I/127I ratio measurements in bovine thyroids from the North Cotentin area (France). Science of The Total Environment 293, 59-67. Frechou, C., and Calmet, D. (2003). 129I in the environment of the La Hague nuclear fuel reprocessing plant--from sea to land. Journal of environmental radioactivity 70, 43-59. Frouin, R., Fiúza, A. F. G., Ambar, I., and Boyd, T. J. (1990). Observations of a Poleward Surface Current off the Coasts of Portugal and Spain During Winter. J Geophys Res 95, 679-691. Fuge, R., and Johnson, C. (1986). The geochemistry of iodine — a review. Environmental Geochemistry and Health 8, 31-54. Gallagher, D., McGee, E., Mitchell, P., Alfimov, V., Aldahan, A., and Possnert, G. (2005). Retrospective Search for Evidence of the 1957 Windscale Fire in NE Ireland Using 129I and Other Long-Lived Nuclides. Environmental science & technology 39, 2927-2935. Gemperline, P. J., Rulifson, R. A., and Paramore, L. (2002). Multi-way analysis of trace elements in fish otoliths to track migratory patterns. Chemometrics and Intelligent Laboratory Systems 60, 135-146. Gentilhomme, V., and Lizon, F. (1997). Seasonal cycle of nitrogen and phytoplankton biomass in a well-mixed coastal system (Eastern English Channel). Hydrobiologia 361, 191-199. Gerzabek, M. H., Muramatsu, Y., Strebl, F., and Yoshida, S. (1999). Iodine and bromine contents of some Austrian soils and relations to soil characteristics. Journal of Plant Nutrition and Soil Science 162, 415-419. Handl, J. (1996). Concentrations of 129I in the biosphere. Radiochimica Acta 72, 33-38. Hansen, V., Roos, P., Aldahan, A., Hou, X., and Possnert, G. (2011a). Partition of iodine (129I and 127I) isotopes in soils and marine sediments. Journal of environmental radioactivity 102, 1096-1104. Hansen, V., Yi, P., Hou, X., Aldahan, A., Roos, P., and Possnert, G. (2011b). Iodide and iodate (129I and 127I) in surface water of the Baltic Sea, Kattegat and Skagerrak. Science of The Total Environment 412–413, 296-303. 63 He, C., Hou, X., Zhao, Y., Wang, Z., Li, H., Chen, N., Liu, Q., and Zhang, L. (2010). 129I level in seawater near a nuclear power plant determined by accelerator mass spectrometer. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. He, P., Aldahan, A., Possnert, G., and Hou, X. L. (2013). A summary of global 129I in marine waters. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 294, 537-541. Herrmann, J., Kershaw, P. j., du Bois, P. B., and Guegueniat, P. (1995). The distribution of artificial radionuclides in the English Channel, southern North Sea, Skagerrak and Kattegat, 1990–1993. Journal of Marine Systems 6, 427-456. HHIN. The Release of Radioactive Materials from Hanford: 1944-1972. The Hanford Health Information Network (HHIN) http://www.doh.wa.gov/hanford/publications/history/release.html. Hoppema, J. M. J. (1991). The oxygen budget of the western Wadden Sea, The Netherlands. Estuarine, Coastal and Shelf Science 32, 483-502. Hou, X., Aldahan, A., Nielsen, S., and Possnert, G. (2009a). Time Series of 129I and 127I Speciation in Precipitation from Denmark. Environ Sci Technol 43, 65226528. Hou, X., Aldahan, A., Nielsen, S., Possnert, G., Nies, H., and Hedfors, J. (2007). Speciation of 129I and 127I in seawater and implications for sources and transport pathways in the north sea. Environ Sci Technol 41, 5993-5999. Hou, X., Chai, C., Qian, Q., Yan, X., and Fan, X. (1997). Determination of chemical species of iodine in some seaweeds (I). Science of The Total Environment 204, 215-221. Hou, X., Dahlgaard, H., and Nielsen, S. (2000a). Iodine-129 time series in Danish, Norwegian and northwest Greenland coast and the Baltic Sea by seaweed. Estuarine, Coastal and Shelf Science 51, 571-584. Hou, X., Dahlgaard, H., and Nielsen, S. (2001). Chemical speciation analysis of 129I in seawater and a preliminary investigation to use it as a tracer for geochemical cycle study of stable iodine. Marine Chemistry 74, 145-155. Hou, X., Dahlgaard, H., Nielsen, S. P., and Ding, W. (2000b). Iodine-129 in human thyroids and seaweed in China. Science of The Total Environment 246, 285-291. Hou, X., Fogh, C., Kucera, J., Andersson, K., Dahlgaard, H., and Nielsen, S. P. (2003). Iodine-129 and Caesium-137 in Chernobyl contaminated soil and their chemical fractionation. The Science of the Total Environment 308, 97-109. Hou, X., Hansen, V., Aldahan, A., Possnert, G., Lind, O. C., and Lujaniene, G. (2009b). A review on speciation of iodine-129 in the environmental and biological samples. Analytica Chimica Acta 632, 181-196. Hou, X., Povinec, P. P., Zhang, L., Shi, K., Biddulph, D., Chang, C.-C., Fan, Y., Golser, R., Hou, Y., Ješkovský, M., et al. (2013). Iodine-129 in Seawater Offshore Fukushima: Distribution, Inorganic Speciation, Sources, and Budget. Environmental science & technology. Hou, X., and Yan, X. (1998). Study on the concentration and seasonal variation of inorganic elements in 35 species of marine algae. Science of The Total Environment 222, 141-156. Hou, X., Yan, X., and Chai, C. (2000c). Chemical Species of Iodine in Some Seaweeds II. Iodine-Bound Biological Macromolecules. Journal of Radioanalytical and Nuclear Chemistry 245, 461-467. Hou, X. L., Dahlgaard, H., Nielsen, S. P., and Kucera, J. (2002). Level and origin of Iodine-129 in the Baltic Sea. Journal of environmental radioactivity 61, 331-343. Howarth, M., and Oceanographic, P. (2001). North Sea Circulation. 64 JAEA Annual report on the effluent control of lowlevel liquidwate in nuclear fuel cycle eneineering laboratories FY 2005,. http://jolissrchinter.tokaisc.jaea.go.jp/pdfdata/JAEA-Review-2006-024.pdf. Jahreis, G., Hausmann, W., Kiessling, G., Franke, K., and Leiterer, M. (2001). Bioavailability of iodine from normal diets rich in dairy products-results of balance studies in women. Experimental and clinical endocrinology & diabetes 109, 163-167. Jia-Zhong, Z., and Whitfield, M. (1986). Kinetics of inorganic redox reactions in seawater: I. The reduction of iodate by bisulphide. Marine Chemistry 19, 121-137. Jickells, T. D., Boyd, S. S., and Knap, A. H. (1988). Iodine cycling in the Sargasso Sea and the Bermuda inshore waters. Marine Chemistry 24, 61-82. Johnson, J., and Stevens, I. (2000). A fine resolution model of the eastern North Atlantic between the Azores, the Canary Islands and the Gibraltar Strait. Deep Sea Research Part I: Oceanographic Research Papers 47, 875-899. Joint, I., and Pomroy, A. (1993). Phytoplankton biomass and production in the southern North Sea. Marine Ecology-Progress Series 99, 169-169. Jones, S. D., and Truesdale, V. W. (1984). Dissolved Iodine Species in a British Freshwater System. Limnology and Oceanography 29, 1016-1024. Küpper, F. C., Carpenter, L. J., McFiggans, G. B., Palmer, C. J., Waite, T. J., Boneberg, E.-M., Woitsch, S., Weiller, M., Abela, R., and Grolimund, D. (2008). Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proceedings of the National Academy of Sciences 105, 6954-6958. Karcher, M. J., I.H. Harms, and Smith, J. N. Long range transport of 129I and 137Cs in the Nordic Seas and the Arctic Ocean. Kekli, A., Aldahan, A., Meili, M., Possnert, G., Buraglio, N., and Stepanauskas, R. (2003). 129I in Swedish rivers: distribution and sources. Science of The Total Environment 309, 161-172. Keogh, S., Aldahan, A., Possnert, G., Finegan, P., Leon Vintro, L., and Mitchell, P. (2007). Trends in the spatial and temporal distribution of 129I and 99Tc in coastal waters surrounding Ireland using Fucus vesiculosus as a bio-indicator. Journal of environmental radioactivity 95, 23-38. Keogh, S. M., Aldahan, A., Possnert, G., Vintró, L. L., Mitchell, P. I., Smith, K. J., and McGinnity, P. (2010). Anthropogenic 129I in precipitation and surface waters in Ireland. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 268, 1232-1235. Kershaw, P. J., McCubbin, D., and Leonard, K. S. (1999). Continuing contamination of north Atlantic and Arctic waters by Sellafield radionuclides. The Science of the Total Environment 237-238, 119-132. Kilius, L., Rucklidge, J., and Soto, C. (1994). The dispersal of 129I from the Columbia River estuary. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 92, 393-397. Krupp, G., and Aumann, D. (1999a). The origin of iodine in soil: I. Iodine in rainfall over Germany. CHEMIE DER ERDE 59, 57-68. Krupp, G., and Aumann, D. C. (1999b). Iodine-129 in rainfall over Germany. Journal of environmental radioactivity 46, 287-299. Kutschera, W., Fink, D., Paul, M., Hollos, G., and Kaufman, A. (1988). Measurement of the 129I/131I ratio in Chernobyl fallout. Physica Scripta 37, 310. López-Gutiérrez, J., Garcı́a-León, M., Schnabel, C., Suter, M., Synal, H. A., Szidat, S., and Garcıá -Tenorio, R. (2004a). Relative influence of 129I sources in a sediment core from the Kattegat area. Science of The Total Environment 323, 195-210. 65 López-Gutiérrez, J. M., Garcı́a-León, M., Schnabel, C., Suter, M., Synal, H. A., and Szidat, S. (2001). Wet and dry deposition of 129I in Seville (Spain) measured by accelerator mass spectrometry. Journal of environmental radioactivity 55, 269-282. López-Gutiérrez, J. M., Santos, F. J., Garcıá -León, M., Schnabel, C., Synal, H. A., Ernst, T., and Szidat, S. (2004b). Levels and temporal variability of 129I concentrations and 129I/127I isotopic ratios in atmospheric samples from southern Spain. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 223–224, 495-500. Lehmann, B. E., Davis, S. N., and Fabryka-Martin, J. T. (1993). Atmospheric and subsurface sources of stable and radioactive nuclides used for groundwater dating. Water Resources Research 29, 2027-2040. Lehto, J., Räty, T., Hou, X., Paatero, J., Aldahan, A., Possnert, G., Flinkman, J., and Kankaanpää, H. (2012). Speciation of 129I in sea, lake and rain waters. Science of The Total Environment 419, 60-67. Luther George, W., Wu, J., and Cullen John, B. (1995). Redox Chemistry of Iodine in Seawater. In Aquatic Chemistry, (American Chemical Society), pp. 135-155. Luther Iii, G. W., and Campbell, T. (1991). Iodine speciation in the water column of the Black Sea. Deep Sea Research Part A Oceanographic Research Papers 38, Supplement 2, S875-S882. Luther Iii, G. W., Ferdelman, T., Culberson, C. H., Kostka, J., and Wu, J. (1991). Iodine chemistry in the water column of the Chesapeake Bay: Evidence for organic iodine forms. Estuarine, Coastal and Shelf Science 32, 267-279. Machín, F., Pelegrí, J. L., Marrero-Díaz, A., Laiz, I., and Ratsimandresy, A. W. (2006). Near-surface circulation in the southern Gulf of Cádiz. Deep Sea Research Part II: Topical Studies in Oceanography 53, 1161-1181. Mauritzen, C., Morel, Y., and Paillet, J. (2001). On the influence of Mediterranean Water on the Central Waters of the North Atlantic Ocean. Deep Sea Research Part I: Oceanographic Research Papers 48, 347-381. Mazé, J. P., Arhan, M., and Mercier, H. (1997). Volume budget of the eastern boundary layer off the Iberian Peninsula. Deep Sea Research Part I: Oceanographic Research Papers 44, 1543-1574. Michel, R., Daraoui, A., Gorny, M., Jakob, D., Sachse, R., Tosch, L., Nies, H., Goroncy, I., Herrmann, J., Synal, H. A., et al. (2012). Iodine-129 and iodine127 in European seawaters and in precipitation from Northern Germany. Science of The Total Environment 419, 151-169. Michel, R., Handl, J., Ernst, T., Botsch, W., Szidat, S., Schmidt, A., Jakob, D., Beltz, D., Romantschuk, L. D., Synal, H. A., et al. (2005). Iodine-129 in soils from Northern Ukraine and the retrospective dosimetry of the iodine-131 exposure after the Chernobyl accident. Science of The Total Environment 340, 35-55. Moisan, T. A., Dunstan, W. M., Udomkit, A., and Wong, G. T. F. (1994). THE UPTAKE OF IODATE BY MARINE PHYTOPLANKTON1. Journal of phycology 30, 580-587. Monitoring our Environment (Sellafield Annual Report). Discharges and Monitoring in the United Kingdom. Sellafield Ltd, United Kingdom. http://sustainability.sellafieldsites.com/environment/environment-page/annualdischarge-monitoring-reports/. Moran, J., Oktay, S., Santschi, P., and Schink, D. (1999). Atmospheric dispersal of 129Iodine from nuclear fuel reprocessing facilities. Environ Sci Technol 33, 2536-2542. 66 Moran, J. E., Fehn, U., and Teng, R. T. D. (1998). Variations in 129I/127I ratios in recent marine sediments: evidence for a fossil organic component. Chemical Geology 152, 193-203. Moran, J. E., Oktay, S. D., and Santschi, P. H. (2002a). Sources of iodine and iodine 129 in rivers. Water Resour Res 38, 1149. Moran, J. E., Oktay, S. D., and Santschi, P. H. (2002b). Sources of iodine and iodine 129 in rivers. Water Resources Research 38, 24-21-24-10. Moreda-Pineiro, A., Romaris-Hortas, V., and Bermejo-Barrera, P. (2011). A review on iodine speciation for environmental, biological and nutrition fields. Journal of Analytical Atomic Spectrometry 26. Muramatsu, Y., and Ohmomo, Y. (1986). Iodine-129 and iodine-127 in environmental samples collected from Tokaimura/Ibaraki, Japan. Science of The Total Environment 48, 33-43. NCRP (1983). Iodine-129: evaluation of releases from nuclear power generation. NCRP Report No.75. Negri, A. E., Fernández Niello, J. O., Wallner, A., Arazi, A., and Steier, P. (2012). Iodine-129 in animal thyroids from Argentina. Science of The Total Environment 430, 231-236. Nichols Jr, R. H., Hohenberg, C. M., Kehm, K., Kim, Y., and Marti, K. (1994). I-Xe studies of the Acapulco meteorite: Absolute I-Xe ages of individual phosphate grains and the Bjurböle standard. Geochimica et cosmochimica acta 58, 25532561. Nies, H., Goroncy, I., Hermann, J., Michel, R., Daraoui, A., Gorny, M., Jakob, D., Sachse, R., Tosch, L., and Nielsen, S. (2010). Kartierung von Tc-99, I-129 und I-127 im Oberflächenwasser der Nordsee. O'Dowd, C. D., Jimenez, J. L., Bahreini, R., Flagan, R. C., Seinfeld, J. H., Hameri, K., Pirjola, L., Kulmala, M., Jennings, S. G., and Hoffmann, T. (2002). Marine aerosol formation from biogenic iodine emissions. Nature 417, 632-636. Oktay, S. D., Santschi, P. H., Moran, J. E., and Sharma, P. (2001). 129I and 127I Transport in the Mississippi River. Environmental science & technology 35, 4470-4476. Oliver, L. L., Ballad, R. V., and Manuel, O. K. (1982). Iodine-129 in Missouri rain and milk. J Radioanal Chem 68, 233-244. Orre, S., Smith, J. N., Alfimov, V., and Bentsen, M. (2010). Simulating transport of 129 I and idealized tracers in the northern North Atlantic Ocean. Environmental fluid mechanics 10, 213-233. Osterc, A., and Stibilj, V. (2008). 127I and 129I/127I isotopic ratio in marine alga Fucus virsoides from the North Adriatic Sea. Journal of environmental radioactivity 99, 757-765. Osterc, A., and Stibilj, V. (2012). Influence of releases of I-129 from reprocessing plants on the marine environment of the North Adriatic Sea. Chemosphere 86, 1020-1027. Paul, M., Fink, D., Hollos, G., Kaufman, A., Kutschera, W., and Magaritz, M. (1987a). Measurement of 129I concentrations in the environment after the Chernobyl reactor accident. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 29, 341-345. Paul, M., Hollos, G., Kaufman, A., Kutschera, W., and Magaritz, M. (1987b). Measurement of 129I concentrations in the environment after the Chernobyl reactor accident. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 29, 341-345. 67 Peliz, Á., Dubert, J., Santos, A. M. P., Oliveira, P. B., and Le Cann, B. (2005). Winter upper ocean circulation in the Western Iberian Basin—Fronts, Eddies and Poleward Flows: an overview. Deep Sea Research Part I: Oceanographic Research Papers 52, 621-646. Persson, S., Aldahan, A., Possnert, G., Alfimov, V., and Hou, X. (2007). 129I Variability in precipitation over Europe. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 259, 508-512. Pham, M., Betti, M., Povinec, P., Alfimov, V., Biddulph, D., Gastaud, J., Kieser, W., López Gutiérrez, J., Possnert, G., Sanchez-Cabeza, J., and Suzuki, T. (2010). Certified reference material IAEA-418: I-129 in Mediterranean Sea water. Journal of Radioanalytical and Nuclear Chemistry 286, 121-127. Pham, M. K., Sanchez-Cabeza, J. A., Povinec, P. P., Arnold, D., Benmansour, M., Bojanowski, R., Carvalho, F. P., Kim, C. K., Esposito, M., Gastaud, J., et al. (2006). Certified reference material for radionuclides in fish flesh sample IAEA414 (mixed fish from the Irish Sea and North Sea). Applied radiation and isotopes 64, 1253-1259. Pingree, R. D. (1993). Flow of surface waters to the west of the British Isles and in the Bay of Biscay. Deep Sea Research Part II: Topical Studies in Oceanography 40, 369-388. Pingree, R. D., and Le Cann, B. (1989). Celtic and Armorican slope and shelf residual currents. Progress in Oceanography 23, 303-338. Povinec, P., Breier, R., Coppola, L., Groening, M., Jeandel, C., Jull, A., Kieser, W., Lee, S. H., Liong Wee Kwong, L., and Morgenstern, U. (2010). Tracing of water masses using a multi isotope approach in the southern Indian Ocean. Earth and Planetary Science Letters. Povinec, P., Oregioni, B., Jull, A., Kieser, W., and Zhao, X. (2000). AMS measurements of 14C and 129I in seawater around radioactive waste dump sites. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 172, 672-678. Qiao, J., Hansen, V., Hou, X., Aldahan, A., and Possnert, G. (2012). Speciation analysis of 129I, 137Cs, 232Th, 238U, 239Pu and 240Pu in environmental soil and sediment. Applied radiation and isotopes 70, 1698-1708. Raisbeck, G., and Yiou, F. (1999). 129I in the oceans: origins and applications. The Science of the Total Environment 237, 31-41. Raisbeck, G., Yiou, F., Zhou, Z., and Kilius, L. (1995). 129I from nuclear fuel reprocessing facilities at Sellafield (UK) and La Hague (France); potential as an oceanographie tracer. Journal of Marine Systems 6, 561-570. Rao, U., and Fehn, U. (1999). Sources and reservoirs of anthropogenic iodine-129 in western New York. Geochimica et cosmochimica acta 63, 1927-1938. Redeker, K. R., Wang, N.-Y., Low, J. C., McMillan, A., Tyler, S. C., and Cicerone, R. J. (2000). Emissions of Methyl Halides and Methane from Rice Paddies. Science 290, 966-969. Reid, J. L. (1978). On the Middepth Circulation and Salinity Field in the North Atlantic Ocean. J Geophys Res 83, 5063-5067. Reith, J. (1930). Der Jodgehalt von Meerwasser. Recueil des Travaux Chimiques des Pays-Bas 49, 142-150. Reithmeier, H., Lazarev, V., Kubo, F., Rühm, W., and Nolte, E. (2005). 129I in precipitation using a new TOF system for AMS measurements. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 239, 273-280. 68 Reithmeier, H., Lazarev, V., Rühm, W., and Nolte, E. (2010). Anthropogenic 129I in the atmosphere: Overview over major sources, transport processes and deposition pattern. Science of The Total Environment 408, 5052-5064. Reithmeier, H., Lazarev, V., Rühm, W., Schwikowski, M., Gäggeler, H., and Nolte, E. (2006). Estimate of european 129I releases supported by 129I analysis in an alpine ice core. Environ Sci Technol 40, 5891-5896. Relvas, P., and Barton, E. D. (2002). Mesoscale patterns in the Cape São Vicente (Iberian Peninsula) upwelling region. J Geophys Res 107, 3164. Richardson, K., and Pedersen, F. B. (1998). Estimation of new production in the North Sea: consequences for temporal and spatial variability of phytoplankton. ICES Journal of Marine Science: Journal du Conseil 55, 574-580. Robens, E., Hauschild, J., and Aumann, D. (1988). Iodine-129 in the environment of a nuclear fuel reprocessing plant: II. Iodine-129 and iodine-127 contents of soils, forage plants and deer thyroids. Journal of environmental radioactivity 7, 265274. Rucklidge, J., Kilius, L., and Fuge, R. (1994). 129I in moss down-wind from the Sellafield nuclear fuel reprocessing plant. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 92, 417-420. Sánchez, R. F., and Relvas, P. (2003). Spring–summer climatological circulation in the upper layer in the region of Cape St. Vincent, Southwest Portugal. ICES Journal of Marine Science: Journal du Conseil 60, 1232-1250. Saiz-Lopez, A., Plane, J., McFiggans, G., Williams, P., Ball, S., Bitter, M., Jones, R., Hongwei, C., and Hoffmann, T. (2006). Modelling molecular iodine emissions in a coastal marine environment: the link to new particle formation. Atmos Chem Phys 6, 883-895. Salomon, J.-C., and Breton, M. (1993). An atlas of long-term currents in the Channel. Oceanologica acta 16, 439-448. Santos, F. J., López-Gutiérrez, J. M., García-León, M., Suter, M., and Synal, H. A. (2005). Determination of 129I/127I in aerosol samples in Seville (Spain). Journal of environmental radioactivity 84, 103-109. Santschi, P., Moran, J., Oktay, S., Hoehn, E., and Sharma, P. 129Iodine: A New Tracer for Surface Water/Groundwater Interaction. Santschi, P. H., and Schwehr, K. A. (2004). 129I/127I as a new environmental tracer or geochronometer for biogeochemical or hydrodynamic processes in the hydrosphere and geosphere: the central role of organo-iodine. Science of The Total Environment 321, 257-271. Schink, D., Santschi, P., Corapcioglu, O., Sharma, P., and Fehn, U. (1995). 129I in Gulf of Mexico waters. Earth and Planetary Science Letters 135, 131-138. Schmidt, A., Schnabel, C., Handl, J., Jakob, D., Michel, R., Synal, H. A., Lopez, J., and Suter, M. (1998). On the analysis of iodine-129 and iodine-127 in environmental materials by accelerator mass spectrometry and ion chromatography. The Science of the Total Environment 223, 131-156. Schmitz, K., and Aumann, D. C. (1995). A study on the association of two iodine isotopes, of natural127I and of the fission product129I, with soil components using a sequential extraction procedure. Journal of Radioanalytical and Nuclear Chemistry 198, 229-236. Schnabel, C., Olive, V., Atarashi-Andoh, M., Dougans, A., Ellam, R. M., Freeman, S., Maden, C., Stocker, M., Synal, H.-A., Wacker, L., and Xu, S. (2007). 129I/127I ratios in Scottish coastal surface sea water: Geographical and temporal responses to changing emissions. Applied Geochemistry 22, 619-627. 69 Schwehr, K. A., and Santschi, P. H. (2003). Sensitive determination of iodine species, including organo-iodine, for freshwater and seawater samples using high performance liquid chromatography and spectrophotometric detection. Analytica Chimica Acta 482, 59-71. Schwehr, K. A., Santschi, P. H., and Elmore, D. (2005). The dissolved organic iodine species of the isotopic ratio of 129I/127I: A novel tool for tracing terrestrial organic carbon in the estuarine surface waters of Galveston Bay, Texas. Limnol Oceanogr: Methods 3, 326-337. Selinus, O. (2005). Essentials of medical geology: impacts of the natural environment on public health: Academic Press). Shinohara, K. (2004). Measurement and behavior of 14C and 129I in the environment. Journal of Radioanalytical and Nuclear Chemistry 260, 265-271. Sive, B. C., Varner, R. K., Mao, H., Blake, D. R., Wingenter, O. W., and Talbot, R. (2007). A large terrestrial source of methyl iodide. Geophysical Research Letters 34, L17808. Smith, J., Jones, E., Moran, S., Smethie Jr, W., and Kieser, W. (2005). Iodine 129/CFC 11 transit times for Denmark Strait overflow water in the Labrador and Irminger seas. Journal of Geophysical Research 110, C05006. Smith, J. D., and Butler, E. C. V. (1979). Speciation of dissolved iodine in estuarine waters. Nature 277, 468-469. Smith, J. N., Ellis, K. M., and Boyd, T. (1999). Circulation features in the central Arctic Ocean revealed by nuclear fuel reprocessing tracers from scientific ice expeditions 1995 and 1996. Journal of Geophysical Research 104, 2966329629,29677. Smith, J. N., Ellis, K. M., and Kilius, L. R. (1998). 129I and 137Cs tracer measurements in the Arctic Ocean. Deep Sea Research Part I: Oceanographic Research Papers 45, 959-984. Smith, J. N., McLaughlin, F. A., Smethie, W. M., Jr., Moran, S. B., and Lepore, K. (2011). Iodine-129, 137Cs, and CFC-11 tracer transit time distributions in the Arctic Ocean. J Geophys Res 116, C04024. Snyder, G., Aldahan, A., and Possnert, G. (2010). Global distribution and long-term fate of anthropogenic 129I in marine and surface water reservoirs. Geochemistry Geophysics Geosystems 11, Q04010. Snyder, G., and Fehn, U. (2004). Global distribution of 129I in rivers and lakes: implications for iodine cycling in surface reservoirs. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 223–224, 579-586. Soldat, J. K., Brauer, F. P., Cline, J. F., Fager, J. E., Klepper, B., Rickard, W. H., Vaughan, B. E., and Watson, D. G. (1973). Radioecology of iodine-129: an interim report. In Other Information: Orig. Receipt Date: 30-JUN-74, p. Medium: ED; Size: Pages: 80. Solomon, S., Garcia, R. R., and Ravishankara, A. R. (1994). On the role of iodine in ozone depletion. Journal of Geophysical Research: Atmospheres 99, 2049120499. Southward, A. J., Langmead, O., Hardman-Mountford, N. J., Aiken, J., Boalch, G. T., Dando, P. R., Genner, M. J., Joint, I., Kendall, M. A., and Halliday, N. C. (2005). Long-term oceanographic and ecological research in the Western English Channel. Advances in Marine Biology 47, 1. Spokes, L. J., and Liss, P. S. (1996). Photochemically induced redox reactions in seawater, II. Nitrogen and iodine. Marine Chemistry 54, 1-10. 70 Suzuki, T., Kabuto, S., Amano, H., and Togawa, O. (2008). Measurement of iodine129 in seawater samples collected from the Japan Sea area using accelerator mass spectrometry: Contribution of nuclear fuel reprocessing plants. Quaternary Geochronology 3, 268-275. Tian, R. C., Marty, J. C., Nicolas, E., Chiavérini, J., Ruiz-Ping, D., and Pizay, M. D. (1996). Iodine speciation: a potential indicator to evaluate new production versus regenerated production. Deep Sea Research Part I: Oceanographic Research Papers 43, 723-738. Truesdale, V. W., Bale, A., and Woodward, E. (2000). The meridional distribution of dissolved iodine in near-surface waters of the Atlantic Ocean. Progress in Oceanography 45, 387-400. Truesdale, V. W. (1978). Iodine in inshore and off-shore marine waters. Marine Chemistry 6, 1-13. Truesdale, V. W., Kennedy, H., Agusti, S., and Waite, T. J. (2003). On the relative constancy of iodate and total-iodine concentrations accompanying phytoplankton blooms initiated in mesocosm experiments in Antarctica. Limnology and Oceanography 48, 1569-1574. Truesdale, V. W., Nausch, G., and Baker, A. (2001). The distribution of iodine in the Baltic Sea during summer. Marine Chemistry 74, 87-98. Truesdale, V. W., and Jones, S. D. (1996). The variation of iodate and total iodine in some UK rainwaters during 1980–1981. Journal of Hydrology 179, 67-86. Tseng, C. L., and Chao, J. H. (1996). Low-level determination of 129I in environmental samples by neutron activation. Applied radiation and isotopes 47, 723-726. Tsunogai, S. (1971). Iodine in the deep water of the ocean. Deep Sea Research and Oceanographic Abstracts 18, 913-919. Tsunogai, S., and Henmi, T. (1971). Iodine in the surface water of the ocean. Journal of the Oceanographical Society of Japan 27, 67-72. Tsunogai, S., and Sase, T. (1969). Formation of iodide-iodine in the ocean. Deep Sea Research and Oceanographic Abstracts 16, 489-496. Ullman, W. J., and Aller, R. C. (1980). Dissolved iodine flux from estuarine sediments and implications for the enrichment of iodine at the sediment water interface. Geochimica et cosmochimica acta 44, 1177-1184. Ullman, W. J., Luther, G. W., Aller, R. C., and Mackin, J. E. (1988). Dissolved iodine behavior in estuaries along the east coast of the United States. Marine Chemistry 25, 95-106. Valkovic, V. (1978). Trace elements in petroleum. Van der Veer, H., and Bergman, M. (1986). Development of tidally related behaviour of a newly settled 0-group plaice (Pleuronectes platessa) population in the western Wadden Sea. Marine Ecology Progress Series 31, 121-129. VanMiddlesworth, L., and Handl, J. (1997). 129I, 131I and 127I in Animal Thyroids after the Chernobyl Nuclear Accident. Health Physics 73, 647-650. Vinogradov, A. P. (1953). The elementary chemical composition of marine organisms. Vogt, R., Sander, R., von Glasow, R., and Crutzen, P. (1999). Iodine Chemistry and its Role in Halogen Activation and Ozone Loss in the Marine Boundary Layer: A Model Study. Journal of Atmospheric Chemistry 32, 375-395. von Glasow, R. (2006). Seaweed, iodine, new particles and atmospheric chemistry— the current state of play. Environmental Chemistry 2, 243-244. 71 Wagner, M. J. M., Dittrich-Hannen, B., Synal, H. A., Suter, M., and Schotterer, U. (1996). Increase of 129I in the environment. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 113, 490-494. Waite, T. J., and Truesdale, V. W. (2003). Iodate reduction by Isochrysis galbana is relatively insensitive to de-activation of nitrate reductase activity—are phytoplankton really responsible for iodate reduction in seawater? Marine Chemistry 81, 137-148. Waite, T. J., Truesdale, V. W., and Olafsson, J. (2006). The distribution of dissolved inorganic iodine in the seas around Iceland. Marine Chemistry 101, 54-67. Wershofen, H., and Aumann, D. C. (1989). Iodine-129 in the environment of a nuclear fuel reprocessing plant: VII. Concentrations and chemical forms of 129I and 127I in the atmosphere. Journal of environmental radioactivity 10, 141-156. Whitehead, D. (1979). Iodine in the UK environment with particular reference to agriculture. Journal of Applied Ecology, 269-279. Wilkins, B. T. (1989). Investigations of iodine-129 in the natural environment : results and implications Wong, G. (1991). The marine geochemistry of iodine. Reviews in Aquatic Sciences 4, 45-73. Wong, G. T. F. (1995). Dissolved iodine across the Gulf Stream Front and in the South Atlantic Bight. Deep Sea Research Part I: Oceanographic Research Papers 42, 2005-2023. Wong, G. T. F., and Cheng, X.-H. (1998). Dissolved organic iodine in marine waters: Determination, occurrence and analytical implications. Marine Chemistry 59, 271-281. Wong, G. T. F., and Cheng, X.-H. (2001a). Dissolved organic iodine in marine waters: role in the estuarine geochemistry of iodine. Journal of Environmental Monitoring 3, 257-263. Wong, G. T. F., and Cheng, X.-H. (2001b). The formation of iodide in inshore waters from the photochemical decomposition of dissolved organic iodine. Marine Chemistry 74, 53-64. Wong, G. T. F., and Hung, C.-C. (2001). Speciation of dissolved iodine: integrating nitrate uptake over time in the oceans. Continental Shelf Research 21, 113-128. Wong, G. T. F., Piumsomboon, A. U., and Dunstan, W. M. (2002). The transformation of iodate to iodide in marine phytoplankton cultures. Marine Ecology Progress Series 237, 27-39. Wong, G. T. F., and Zhang, L.-S. (2008). The kinetics of the reactions between iodide and hydrogen peroxide in seawater. Marine Chemistry 111, 22-29. Yi, P., Aldahan, A., Hansen, V., Possnert, G., and Hou, X. L. (2010). Iodine Isotopes (129I and 127I) in the Baltic Proper, Kattegat, and Skagerrak Basins. Environmental science & technology 45, 903-909. Yi, P., Aldahan, A., Possnert, G., Hou, X. L., Hansen, V., and Wang, B. (2012a). 127I and 129I Species and Transformation in the Baltic Proper, Kattegat, and Skagerrak Basins. Environmental science & technology 46, 10948-10956. Yi, P., Aldahan, A., Possnert, G., Hou, X. L., He, P., and Wang, B. (2012b). Seasonal variation of 129I species in the Baltic Proper. Journal of Radioanalytical and Nuclear Chemistry, 1-5. Yi, P., Possnert, G., Aldahan, A., Hou, X. L., Bryhn, A. C., and He, P. (2013). 129I in the Baltic Proper and Bothnian Sea: application for estimation of water exchange and environmental impact. Journal of environmental radioactivity 120, 64-72. 72 Yiou, F., Raisbeck, G. M., Christensen, G. C., and Holm, E. (2002). 129I/127I, 129I/137Cs and 129I/99Tc in the Norwegian coastal current from 1980 to 1998. Journal of environmental radioactivity 60, 61-71. Yoshida, Y. M., Satoshi (1999). Effects of microorganisms on the fate of iodine in the soil environment. Geomicrobiology Journal 16, 85-93. Yuita, K. (1992). Dynamics of iodine, bromine, and chlorine in soil: II. Chemical forms of iodine in soil solutions. Soil Science and Plant Nutrition 38, 281-287. Zenk, W. (1970). On the temperature and salinity structure of the Mediterranean water in the Northeast Atlantic. Deep Sea Research and Oceanographic Abstracts 17, 627-631. Zimmerman, J. T. F. (1976). Mixing and flushing of tidal embayments in the western Dutch Wadden Sea part I: Distribution of salinity and calculation of mixing time scales. Netherlands Journal of Sea Research 10, 149-191. 73 Acta Universitatis Upsaliensis Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1068 Editor: The Dean of the Faculty of Science and Technology A doctoral dissertation from the Faculty of Science and Technology, Uppsala University, is usually a summary of a number of papers. A few copies of the complete dissertation are kept at major Swedish research libraries, while the summary alone is distributed internationally through the series Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology. Distribution: publications.uu.se urn:nbn:se:uu:diva-206711 ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2013