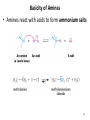* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 1 Chemical Bonding and Chemical Structure
Survey
Document related concepts
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Kinetic resolution wikipedia , lookup
Aromaticity wikipedia , lookup
Hydroformylation wikipedia , lookup
Elias James Corey wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Bottromycin wikipedia , lookup
Discodermolide wikipedia , lookup
Transcript
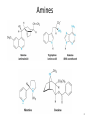
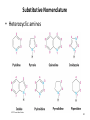
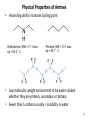
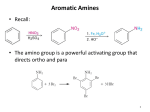
Chapter 23 The Chemistry of Amines Amines 2 skatole 3 Classification of Amines • Amines are organic derivatives of ammonia • Note how amines are classified differently from that of alcohols 23.1 Nomenclature of Amines 4 Common Nomenclature • For simple amines, add “amine” to the name of the alkyl group • When alkyl groups differ, name the amine as an Nsubstituted amine 5 Common Nomenclature • Aromatic amines are named as derivatives of aniline 23.1 Nomenclature of Amines 6 Substitutive Nomenclature • Amines are named in a similar fashion to the their analogous alcohols – Remove the “-e” and replace it with “-amine” • If there is more than one amino group, use Greek prefixes to indicate how many and numbers to indicate location on the alkyl chain 23.1 Nomenclature of Amines 7 • When alkyl groups differ, use the Nsubstitution system • When there is more than one substituted amine, use N and N’ to differentiate 8 • Name the amino group as a substituent when there is a higher priority principal group • Recall: Substitutive Nomenclature • Heterocyclic amines 10 Problems 1) Name the following compounds: 11 2) Draw the following molecules • 2-propen-1-amine • N-methyl-N’-propyl-1,4-butanediamine • p-aminophenol 12 Structure of Amines • Bond length: • Most amines undergo rapid inversion at N 23.2 Structure of Amines 13 Physical Properties of Amines • H-bonding ability increases boiling point Diethylamine, MW = 71.1 amu bp = 56.3°C Pentane, MW = 72.1 amu bp = 36.1°C • Low molecular weight amines tend to be water soluble whether they are primary, secondary or tertiary • Fewer than 5 carbons usually = solubility in water 14 15 Basicity of Amines • Amines react with acids to form ammonium salts 16 Separations Using Amine Basicity • Ammonium salts are ionic compounds which imparts a high degree of water-solubility • This property can be useful in separation of amines from other compounds Acidity of Amines • NH3, RNH2, and R2NH are amphoteric: they may act as bases and acids • They are very weakly acidic – Will give up H+ to a very strong base • The conjugate base of an amine is called an amide (do not confuse with amide derivatives of carboxylic acids) 18 Quaternary Salts • Quaternary ammonium and phosphonium salts are compounds in which all four groups around the N and P are alkyl or aryl 23.6 Quaternary Ammonium and Phosphonium Salts 19 Phase-Transfer Catalysis 20 Synthesis of Amines 21 Direct Alkylation of Amines • Further alkylations can take place to give complex mixtures Gabriel Synthesis of Primary Amines • Direct alkylation is not a good method for the preparation of primary amines • The Gabriel synthesis allows for controlled preparation 23.11 Synthesis of Amines 23 • Hydrolysis of the phthalimide frees the amine 23.11 Synthesis of Amines 24 Reduction of Nitro Compounds • Catalytic hydrogenation: • Reduction with finely divided metal powders: 23.11 Synthesis of Amines 25 Reduction of Nitro Compounds • LiAlH4 and NaBH4 fail to provide the amine 23.11 Synthesis of Amines 26 Reductive Amination 27 28 • Formaldehyde can be used to introduce methyl groups • Neither an imine nor an enamine can be an intermediate in the reaction of a secondary amine and formaldehyde 23.7 Alkylation and Acylation Reactions of Amines 30