* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 10. Chemical Bonding II. Molecular Geometry and
Hartree–Fock method wikipedia , lookup
Biochemistry wikipedia , lookup
Hydrogen bond wikipedia , lookup
Atomic nucleus wikipedia , lookup
Halogen bond wikipedia , lookup
Electronegativity wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Spin crossover wikipedia , lookup
Aromaticity wikipedia , lookup
Computational chemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic orbital wikipedia , lookup
Bond valence method wikipedia , lookup
Metallic bonding wikipedia , lookup
Electron configuration wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
History of molecular theory wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Chemical bond wikipedia , lookup
Molecular orbital wikipedia , lookup
Chapter 10. Chemical Bonding II. Molecular Geometry
and Hybridization of Atomic Orbitals
10.1 Molecular Geometry (what, how, why)
General Summary -- Structure and Bonding Concepts
Octet Rule
Electronic Configuration
of Atoms
Lewis Electron Dot
Formula of Molecule
VSEPR Theory
3-D Shape of Molecule
Electronegativity
and Bond Polarity
Polarity of Molecule
Intermolecular Forces
and Bulk Properties
Valence Bond
Theory
Bonding Description
of Molecule
Chemical Reactivity
Valence Shell Electron Pair Repulsion Theory
Hypothesis --
The structure of a molecule is that which minimizes the
repulsions between pairs of electrons on the central atom.
"Effective Number" (EN) = (number of atoms attached to central atom)
+ (number of lone pairs on central atom)
(Using the number of atoms is simpler than the number of bonding pairs, because
this accounts for double and triple bonds which essentially occupy the same space
as a single bond.)
EN
Arrangement of Electron Pairs
linear
180°
2
3
trigonal planar
120°
tetrahedral
109.5°
4
e
e
e
trigonal
bipyramidal
120° & 90°
a
6
Examples
linear
BeCl2, CO2
trigonal planar
BCl3, CH3+
bent
SnCl2, NO2-
tetrahedral
pyramidal
CH4, PO43NH3, ClO3-
bent
H2O, SeF2
trigonal bipyramid
a
5
Molecular Shape
(Geometry)
"see saw"
T-shaped
linear
ClF3, XeO32XeF2, ICl2-
square pyramid
SF6, PCl6BrF5, SF5-
square planar
XeF4, IF4-
octahedral
octahedral
90°
PF5, SeCl5+
SF4, BrF4+
Important corollaries:
•
In trigonal bipyramid structures, lone e- pairs adopt equatorial positions (e)
•
Order of repulsions: Lp - Lp > Lp - Bp > Bp - Bp
(Predicts distortions from ideal geometries)
Molecules with more than one central atom, e.g., CH3OH, methanol
H
H
C
H
•
•
•
O
H
C and H are "central"
HCH and OCH ∠ 109 °
O is like water with two lone pairs and two bonding pairs: ∠HOC = 105
10.2 Dipole Moments (Polarity of Molecules)
predict from molecular shape
A. Bond Polarity
e.g., HF molecule
• F is more electronegative than H, so there is partial charge
separation in the H-F bond:
δ+ δ−
H F
•
or
H
F
the H-F bond is described as "polar covalent bond" and is said to
have a "dipole moment"
B. Molecule Polarity
(Polar or Non-Polar?)
•
Polar bonds do not always mean the molecule is polar
•
In very symmetrical structures (e.g., CO2 or CF4), the
individual bond dipoles effectively cancel each other and the
molecule is non-polar.
F
O
C
O
F
C
F
F
•
In less symmetrical structures (e.g., SO2 and SF4), the
bond dipoles do not cancel and there is a net dipole moment
which makes the molecule polar.
F
..
O
S
F
S
O
F
F
..
Other examples for practice:
Polar:
H2O
Non-Polar:
SnCl2
BeCl2
NH3
CH4
SeF2
PF5
PF3
XeF2
BrF5
XeF4
10.3 Valence Bond Theory
A.
Why is additional theory needed?
•
VSEPR predicts H2 and F2 are the same type of bond
•
•
•
H2 bond dissociation energy = 436.4 kJ/mole
F2 bond dissociation energy = 150.6 kJ/mole
Need better theory to explain these types of differences.
XeO3
SO3
B.
Basic Concept
Covalent Bonds result from overlap of atomic orbitals
consider the H2 molecule
1s
.
H
1s
+
σ bond
.
. .
H
H2
Figure 10.5 shows the change in potential energy with distance
between atoms
• atoms distant - no interaction
• atoms approach - electron from one atom is attracted to
nucleus of other
• atoms get very close - nuclei repel each other
F2 molecule
2p
σ bond
2p
.
.
+
F
.
.
F
F2
HF molecule
.
2p
+
H
σ bond
.
. .
F
H
F
Two types of covalent bonds:
σ (sigma) bond:
"head-to-head" overlap along the bond axis
(as in previous pictures)
π (pi) bond:
"side-to-side" overlap of p orbitals:
π bond
.
2p
+
.
. .
2p
•
single bond -- always a σ bond
•
double bond -- combination of one σ bond and one π bond
•
triple bond -- combination of one σ bond and two π bonds
10.4 Hybridization of Atomic Orbitals
Problem:
•
•
•
Describe the bonding in CH4 molecule.
experimental fact -- CH4 is tetrahedral (H-C-H angle = 109.5°)
VSEPR theory "explains" this with 4 e- pairs, ∴ tetrahedral
however, if only s and p orbitals are used, the angles ought to be 90°
since the p orbitals are mutually perpendicular!
Solution:
Modify the theory of atomic orbitals and use:
Hybridization: combination of 2 or more atomic orbitals on
the same atom to form a new set of
"Hybrid Atomic Orbitals" used in bonding.
CH4
(methane)
Energy Level Diagram
C
↑↓
2s
↑
2p
↑
2p
2p
ground state - valence shell orbital diagram
• H electrons can only share with C p orbitals
•
So try promoting
C
↑
2s
↑
2p
↑
2p
↑
2p
bond angles can only be 90° angles required
by p orbitals -- wrong, must be 109°
Now have four orbitals, but still have three that
are 90° apart
So try mixing the four into new orbitals
C
↑
↑
↑
↑
hybridized state (mixing of s and p orbitals)
3
3
3
3
sp sp sp sp
(gives 4 identical bonds that are 109.5° angles
-- right!)
So what do these new orbitals look like?
see Figures 10.7 and 10.8 and 10.9
Types of Hybrid Orbitals (see Table 10.4)
Atomic Orbitals
Hybrid Orbitals
Geometry
linear
(180°)
trigonal planar
(120°)
tetrahedral
(109.5°)
one s + one p
two sp
one s + two p
three sp2
one s + three p
four sp3
one s + three p +
one d
five dsp3
trigonal bipyramid
90° & 120°
one s + three p +
two d
six d2sp3
octahedral
90°
Unhybridized p
Orbitals (left over)
2
1
0
{ Note: combination of n AO's yields n Hybrid Orbitals)
Practice:
Use valence bond theory to describe the bonding in H2O, NH3, CH4, PF3.
Draw clear 3-D pictures (method shown in class) showing orbital overlap, etc.
[Note: All use only simple σ bonds and lone pairs.]
10.5 Hybridization in Molecules Containing Double & Triple Bonds
ethylene: sigma + pi bond
H
H
C
H
C
H
(see Figure 10.16)
acetylene sigma + two pi bonds
(see Figure 10.19)
PRACTICE on
• H2CNH
• HCN
(use double bond like H2CO and H2CCH2)
(use triple bond like HCCH)
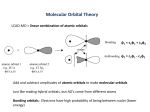
10.6 Molecular Orbital Theory
A. Comparison of VB and MO Theory
Valence Bond Theory ("simple" but somewhat limited)
•
e- pair bonds between two atoms using overlap of atomic orbitals
on two atoms
•
sometimes fails to explain facts, e.g., O2 is paramagnetic
indicating unpaired electrons, but VB theory would indicate that
electrons in the σ and π orbitals are paired.
Molecular Orbital Theory (more general but "complex")
•
all e-'s in molecule fill up a set of molecular orbitals that are
made up of linear combinations of atomic orbitals on two or more
atoms
MO's can be:
•
•
"localized" -- combination of AO's on two atoms, as in
the
diatomic molecules
"delocalized" -- combination of AO's on three or more
atoms, as in benzene (C6H6) -- page 424
B. Bonding and Antibonding Molecular Orbitals
Molecular Orbitals for simple diatomic molecules (H2 and He2)
in H2 the 1s atomic orbitals on the two H atoms are combined into:
•
•
a bonding MO -- σ1s
• lower energy than the atomic orbitals from which it
was formed, .i.e., greater stability
• more electron density between nuclei from
constructive interference (wave properties of the
electron)
an antibonding MO -- σ*1s
• higher energy and lower stability than the atomic
orbitals from which it was formed
• no electron density between nuclei from
destructive interference
MO energy level diagram for H2 (only the bonding MO is filled):
σ*1s
1s
1s
σ1s
H
H2
H
Figure 10.23
In contrast, the MO diagram for the nonexistent molecule, He2 shows that
both bonding and antibonding MO's are filled:
σ*1s
1s
1s
σ1s
He
He2
He
Figure 10.25
Bond Order = ½ [(# bonding e-'s) - (# antibonding e-'s)]
for H2
= ½ [2 - 0] = 1
(a single bond)
for He2
= ½ [2 - 2] = 0
(no net bonding interaction)
C.
Bonding and Antibonding Molecular Orbitals from p Atomic Orbitals
Figure 10.24 - shows interaction to form
• σ bonds when atomic orbitals approach end to end
• π bonds when atomic orbitals approach side to side
10.7
Molecular Orbital Configurations/Rules
A. Rules
1. The number of MO's equal the number of AO's used to make the
MO's
2. The more stable the bonding MO, the less stable the antibonding
MO
3. MO's fill from low to high energies
4. In stable molecules, the number of
electrons in bonding MO's > electrons in antibonding MO's
5. Maximum of 2 electrons per MO (with opposite spins)
6. Follow Hund's rule - electrons do not pair until all MO's of the same
energy are half filled
7. The number of electrons in MOs equals sum of all of the electrons
in the atoms
B. MO's for 2nd Row Diatomic Molecules (e.g., N2, O2, F2, etc.)
MO energy level diagram -- Figures10.26 plus 10.27
σ*2p x
π*2p y
π*2p z
2p
2p
σ2p x
π2p
π2pz
y
σ*2s
2s
2s
σ2s
σ*1s
1s
1s
σ1s
Examples -- Table 10.5 (Note the change at O2 and F2)
Fill in MO diagram for C2, N2, O2, F2, and Ne2
and determine bond order for each:
molecule
C2
N2
O2
F2
Ne2
bond order
2
3
2
1
0
B.
Electron Configurations
valence electrons in blue
Example: N2
(σ1s)2(σ*1s)2(σ2s)2(σ*2s)2(π2p )2(π2p )2(σ2p )2
y
z
x
Example: O2
(σ1s)2(σ*1s)2(σ2s)2(σ*2s)2 (σ2p )2 (π2p )2(π2p )2 (π*2p )1(π*2p )1
x
y
z
y
z
10.7 Delocalized Molecular Orbitals
By combining AO's from three or more atoms, it is possible to generate MO's
that are "delocalized" over three or more atoms
Examples:
Resonance in species like HCO32-, CO3-2 and benzene (C6H6)
can be "explained" with a single MO description containing
delocalized π bonds.
•
see Figure 10.28 sigma framework in benzene
H
H
H
C
C
C
C
C
C
H
H
H
•
•
see Figure 10.29 for pi framework
see Figure 10.30 for the carbonate ion pi framework
Computer model:: HIV enzyme with buckeyball occupying the active site preventing enzyme
from working as it usually does.
Carbonate Ion
Bonding in Solids -- Band Theory
An energy "band" is composed of a very large number of closely spaced
energy levels that are formed by combining similar atomic orbitals of atoms
throughout the substance
Metals and metalloids have a
•
"conduction band" -- set of highly delocalized, partially filled, MO's that
extend over the entire solid lattice structure
•
"band gap" -- energy difference between filled "valence band"
and the conduction band (Figure 20.10)