* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Single Gene Inheritance - Ms. Pallante
Survey
Document related concepts
Gene expression programming wikipedia , lookup
Polymorphism (biology) wikipedia , lookup
Genomic imprinting wikipedia , lookup
Public health genomics wikipedia , lookup
Inbreeding avoidance wikipedia , lookup
Medical genetics wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Genome (book) wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
X-inactivation wikipedia , lookup
Population genetics wikipedia , lookup
Designer baby wikipedia , lookup
Microevolution wikipedia , lookup
Genetic drift wikipedia , lookup
Hardy–Weinberg principle wikipedia , lookup
Transcript
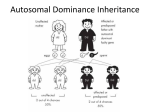
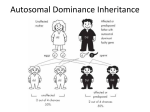
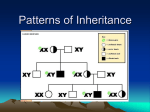
Patterns of Single Gene Inheritance I & II Virginia A. Pallante, M.S. REQUIRED READING Nussbaum, RL, McInnes, RR, and Willard HF (2007) Thompson and Thompson Genetics in Medicine, 7th edition. Saunders: Philadelphia. Chapter 7, pp. 115 135. Complete problems 1 6 and 8 from Chapter 7, and problems 5 & 6 from Chapter 11. LECTURE OBJECTIVES 1. Describe and provide examples of: a. Simple patterns of Mendelian inheritance encountered in clinical practice. b. Aspects of phenotypic expression. 2. Illustrate how single gene inheritance patterns may be deduced from pedigree analysis. 3. Discuss basic concepts of probability and show how to calculate the probability of offspring genotypes given parental mating types. STUDENT OBJECTIVES 1. Describe the general features of Mendelian patterns of single gene inheritance. 2. Identify the mode of inheritance of traits discussed in lecture. 3. Describe aspects of phenotypic expression, using traits discussed in lecture as examples. 4. Understand basic concepts of probability. 5. Recognize the pattern of inheritance of a trait segregating in a family. 6. Apply basic concepts of probability and principles of Mendelian inheritance to calculate the probabilities that offspring of specified mating types will be affected and unaffected. IMPORTANT TERMS locus allele genotype phenotype autosomal Xlinked penetrance codominant dominant recessive homozygous heterozygous hemizygous expressivity compound heterozygote carrier (obligate heterozygote) genetic heterogeneity pleiotropy age of onset sexlimited sexinfluenced I. II. III. The Gene is the Unit of Inheritance A. The location of a gene on a chromosome is its locus. B. Alternative forms of a gene at a particular locus are referred to as alleles. C. An individual’s genotype (genetic composition) at a particular locus is defined by the nature of the alleles at that locus. 1. If both alleles are identical, then the individual is homozygous at the locus. Homozygosity may refer to the presence of two normal or two mutant alleles. 2. If the alleles differ, then the individual is heterozygous at the locus. If two different mutant alleles are present, then the individual is a compound heterozygote. D. The genotype at a particular locus and the environment in which it is expressed determines the phenotype or observed characteristics of an individual. E. Traits that are determined by loci on one of the 22 autosomes are autosomal. Traits determined by loci on the X chromosome are Xlinked, and those determined by loci on the Y chromosome are Ylinked. Gregor Mendel’s Laws of Inheritance A. Law of Unit Inheritance parental characteristics do not blend because there is a unit of inheritance. Mendel’s “units” are now known as genes or alleles. B. Law of Segregation the two alleles at a particular locus segregate into different gametes. C. Law of Independent Assortment alleles at different loci are transmitted independently of each other. Linkage is an exception to this rule. Dominant and Recessive Inheritance A. Nomenclature: For dominant traits the capital letter (e.g. A) represents the mutant allele and the small letter (e.g. a) represents the normal allele. For recessive traits, the small letter (e.g. a) represents the mutant allele and the capital letter (e.g. A) represents the normal allele. IV. B. Autosomal dominant traits are those traits in which the phenotype of the heterozygote and the homozygote for the dominant allele are the same, i.e., Aa and AA have the same phenotype where A=dominant allele. These traits are expressed when only one copy of the dominant allele is present. In practice, if the heterozygote expresses the trait, then the trait is classified as dominant, even if the phenotype of the homozygote (AA) and heterozygote (Aa) are different. C. Autosomal recessive traits are those traits in which the phenotype is expressed only if homozygous for the recessive allele, i.e., aa where a=recessive allele. Two copies of the recessive allele are necessary for expression. D. If the heterozygote (AB) has a different phenotype than either of the homozygotes (AA or BB), then the alleles are said to be codominant. E. Xlinked dominant traits are those expressed when either males or females have one copy of the dominant allele, i.e., X A Y or X A X a where A=dominant allele. F. Xlinked recessive traits are those expressed in males who carry one copy of the recessive allele (i.e., are hemizygous, X a Y where a=recessive allele). Two copies of the recessive allele are generally required for females to express the trait, i.e., X a X a . Examples and Features of Autosomal Dominant Inheritance A. Examples: familial hypercholesterolemia, Huntington disease, neurofibromatosis type I (NF1), myotonic dystrophy, Marfan syndrome and achondroplasia (see description of disorders at the end of the syllabus). B. Features of dominant conditions 1. 2. 3. 4. 5. 6. 7. Vertical inheritance direct transmission from grandparent to parent to child without skipping generations. Both sexes are affected in a 1:1 ratio. Both sexes may transmit the trait. In some cases, variable expressivity (i.e., varying levels of severity) and variable age of onset (i.e., varying age when symptoms present). Heterozygotes are much more common than homozygotes In some cases, homozygotes are more severely affected than heterozygotes (e.g., familial hypercholesterolemia). May result from a new mutation causing a sporadic case (i.e. no family history of disorder). 8. C. The gene product is usually a nonenzymatic (structural) protein. Transmission probabilities and the use of the Punnett square. 1. If one parent has the disorder (assumed to be Aa) and the other does not (aa) then there is a 50% chance that the child will inherit the disorder and a 50% chance that they will not. 2. If both parents have the disorder (assumed to be Aa x Aa) then there is a 75% chance that their children will inherit the disorder, and a 25% chance that they will not. Figure 1 Figure 2 Source: Virginia Pallante at Virginia Commonwealth University School of Medicine V. Examples and Features of Autosomal Recessive Inheritance A. Example disorders include cystic fibrosis, sickle cell anemia, TaySachs disease, phenylketonuria, and most inborn errors of metabolism. B. Affected homozygotes are commonly the offspring of two heterozygote carriers. C. Features of recessive conditions: 1. 2. 3. 4. 5. D. Transmission probabilities and use of the Punnett square 1. 2. E. Horizontal inheritance affected individuals often occur within the same sibship or generation. Both sexes are affected in a 1:1 ratio. For rare recessive disorders consanguinity or genetic relatedness may be observed in the parents of affected offspring. Both sexes are equally capable of transmitting the mutant allele, and usually both parents are established as carriers with the birth of a child with the disorder. The gene product is usually an enzymatic protein. If both parents are carriers (Aa x Aa) then there is a · 25% chance that the child will have the disorder (aa) · 50% chance that the child will be a carrier (Aa), and · 25% chance that the child will be neither affected nor a carrier (AA). Thus the chance that an unaffected child of carrier parents is also a carrier is two in three. Autosomal recessive phenotypes in local populations. 1. 2. 3. Sickle cell anemia in individuals of African descent. TaySachs disease in the Ashkenazi Jewish and French Canadian populations Cystic fibrosis in northern European Caucasians. Figure 3 Source: Virginia Pallante at Virginia Commonwealth University School of Medicine Figure 4 Figure 5 Source: Virginia Pallante at Virginia Commonwealth University School of Medicine VI. Sex Linkage and XInactivation A. Dosage compensation 1. 2. B. Xinactivation in females allows compensation for this difference in dosage for Xlinked traits. 1. 2. 3. 4. VII. For autosomal traits, two doses lead to a normal phenotype, while one dose or more than two doses often have clinical significance. For Xlinked traits two doses in females and one dose in males both lead to a normal phenotype. Lyon hypothesis In early embryonic life (37 days after fertilization) one X chromosome is inactivated. The inactive X chromosome is condensed in a Barr body. Inactivation of the maternal or paternal X chromosome is random, but once it occurs, the same X will be inactive in all descendants of a particular cell. Some genes on the inactive X chromosome remain active, i.e., escape inactivation. These include the genes in the pseudoautosomal region that have matching genes on the Y chromosome, genes outside the pseudoautosomal region that have related copies on the Y chromosomes, and others. Examples and Features of XLinked Recessive Inheritance A. Example disorders include Hemophilia A and Duchenne muscular dystrophy. B. Features of recessive Xlinked conditions: 1. 2. 3. 4. 5. C. Transmission probabilities and use of the Punnett square 1. 2. 3. 4. Figure 6 Incidence of trait much higher in males than in females. Absence of maletomale transmission. Diagonal inheritance affected males within a family are related through females of the maternal line. Full expression in hemizygous males who have inherited the mutant allele from their mother. No or occasional mild expression in females due to Xinactivation. A son never inherits the disorder from his father. All daughters of a male with the disorder are obligate carriers. Sons of carrier females have a 50% chance of inheriting the disorder. Daughters of carrier females have a 50% chance of being carriers too. Figure 7 Source: Virginia Pallante at Virginia Commonwealth University School of Medicine Figure 8 Source: Virginia Pallante at Virginia Commonwealth University School of Medicine VIII. Examples and Features of XLinked Dominant Inheritance A. B. Example disorder: Vitamin D resistant rickets, Rett syndrome (usually lethal in hemizygous males) . Features of dominant Xlinked conditions 1. 2. 3. 4. Twice as many females with the disorder as males. An absence of maletomale transmission. Males with the disorder transmit it to all daughters and no sons. Females usually have more mild and variable expression of the disorder due to Xinactivation. 5. C. Transmission probabilities and use of the Punnett square 1. 2. 3. 4. 5. Figure 9 Few disorders classified as Xlinked dominant. A son never inherits the disorder from his father. All daughters of male with the disorder will also have the disorder. Sons of affected females have a 50% chance of inheriting the disorder. Daughters of affected females also have a 50% chance of inheriting the disorder. Can distinguish between autosomal and Xlinked dominant by looking at offspring of affected males. Figure 10 Source: Virginia Pallante at Virginia Commonwealth University School of Medicine IX. Phenotypic Expression A. Penetrance refers to the all or none expression of a mutant genotype. It usually refers to dominant traits in heterozygotes, and means that even though an individual has inherited the mutant allele, there may be no expression of the phenotype. If a condition is expressed in less than 100 percent of persons who have one copy of the mutant allele, it is said to have reduced penetrance. Examples of reduced penetrance: 1. Retinoblastoma, a malignant eye tumor. About 10% of individuals who transmit the mutant allele are unaffected. Therefore, the mutant allele is 90% penetrant. 2. Waardenburg syndrome: congenital sensorineural deafness, heterochromia, displacement of the inner canthi, white forelock, and other features. Since only about 20% of people with Waardenburg syndrome are deaf, this shows reduced penetrance of this feature of this syndrome. B. Expressivity is the extent to which a genetic defect is expressed. With variable expressivity, a trait may vary in expression from mild to severe. However, it is never completely unexpressed. Examples: neurofibromatosis, myotonic dystrophy. C. Variable age of onset refers to the variation in the time to phenotypic expression of mutant gene (s). Example: the onset of Huntington disease is typically in the 40’s, however, age of onset may range from 15 to 70+ years. D. A mutant gene is said to be pleiotropic when it produces a wide range of phenotypic effects. Example: Marfan syndrome involves the skeletal, cardiovascular, and ocular systems. E. Genetic heterogeneity includes both locus heterogeneity and allelic heterogeneity. 1. Locus heterogeneity is when mutations at two different genetic loci result in similar phenotypes (Example: congenital deafness). In some cases, the mode of inheritance of the disorders can vary. 2. Allelic heterogeneity refers to two or more different mutant alleles at the same genetic locus (Example: Duchenne and (the less severe) Becker muscular dystrophy; cystic fibrosis). F. Sexlimited refers to a phenotype that is autosomally transmitted but expressed only in one sex. Example: Autosomal dominant male precocious puberty. G. Sexinfluenced refers to autosomally inherited traits that are expressed differently, in either degree or frequency, in males and females. Example: hemochromatosis (autosomal recessive disorder of increased absorption of dietary iron) is more commonly found in males due to lower dietary intake and menstruation in females. H. The distinction between single gene and complex, multigene disorders is blurring as we continue to learn about genetics. For an excellent article that discusses this topic please see “Heterozygosity for a Mendelian disorder as a risk factor for complex disease” by E. Sidransky in Clin Genet (2006) 70:275 282. ADDITIONAL RESOURCES (Optional) Mawer, Simon. Mendel’s Dwarf. New York, Harmony Books, 1998. An “imaginative and intelligent novel” which tells the story of a research scientist who happens to have achondroplasia and is a distant relative of Gregor Mendel. Mendel Web a teaching and learning resource built upon Gregor Mendel's famous paper of 1865. www.mendelweb.org Literature, Arts, and Medicine Database annotated bibliography of prose, poetry, film, video and art developed to be used as a resource in medical humanities. http://endeavor.med.nyu.edu/litmed/litmeddb APPENDIX Basic Concepts of Probability Probability: Probability is used to determine the chance of an outcome occurring in any one trial. It is equal to the expected proportion of an outcome in a series of events. Example: Outcome: Xbearing or Ybearing sperm Events: All sperm in an ejaculate Trial: Fertilization of a single egg Probability:1/2 for X, 1/2 for Y Law of Independence: Applies if the occurrence of an outcome in one trial does not influence the probability of another outcome in a subsequent trial. Example: Given that a couple has had one boy, the probability that their next offspring is male is still 1/2. Multiplication Rule: The combined probability of two or more independent outcomes happening in two or more trials is the product of their individual probabilities. [a and b multiply] Example: The probability of a couple having two boys in row is 1/2 x 1/2 = 1/4. Addition Rule: The probability of two or more alternative outcomes happening in the same trial is the sum of their individual probabilities. [a or b add] Example: The probability of a couple having either or boy or a girl is 1/2 + 1/2 = 1. Application of Probability I Q. Waardenburg syndrome is an autosomal dominant condition that accounts for 1.4 percent of congenitally deaf persons. Given that a woman has Waardenburg syndrome (and is assumed to be a heterozygote) and a man is unaffected, what is the probability that none of their three children will be affected? A. The multiplication rule is used to compute the joint probability of three children being unaffected, since the birth of one unaffected child is independent of the birth of another unaffected child. The probability of any one child being unaffected is 1/2. Thus, the probability of all three remaining unaffected is 1/2 x 1/2 x 1/2 = 1/8. Application of Probability II Q. An unrelated man and woman both have a sibling affected with cystic fibrosis. The man and woman are phenotypically normal. Their parents are unaffected with CF, but are assumed to be carriers. What is the probability that a child of this couple will be affected with CF? A. The multiplication rule is used to compute the joint probability that the man is a CF carrier, the woman is a CF carrier, and (given that they both are CF carriers), the probability that their child is affected. This can be written as: Pman, carrier x Pwoman, carrier x Paffected child/parental genotypes Pman, carrier and Pwoman, carrier are derived from the Punnett square showing the mating of two heterozygotes. Since neither the man nor the woman are affected, this outcome of their parents’ mating can be ruled out. Thus, for both the man and woman, there remains a 2/3 chance that they carry the mutant allele. Paffected child/parental genotypes is also derived from the Punnett square mating of two heterozygotes and is equal to 1/4. Thus, the overall probability is: 2/3 x 2/3 x 1/4 = 4/36 =1/9 Application of Probability III Q. One type of retinititis pigmentosa (RP) (a disorder characterized by retinal defects) is inherited as an Xlinked recessive trait. A man with RP mates with a healthy woman whose father had RP. Assuming complete penetrance of the RP mutant allele, what is the probability that a child of theirs would have RP? A. This problem can be answered using either the Punnett square approach or the multiplication and addition rules of probability. In both cases, the problem is complicated by the fact that the recurrence risk of RP depends upon the sex of the child. Therefore, we must consider the chance that the child is a male and has RP or the chance that the child is a female and has RP. Using the addition and multiplication rules of probability this problem can be written as: Pmale x Paffected male + Pfemale x Paffected female We know that: Pmale = Pfemale = 1/2 Since the woman’s father had RP, she must be a carrier. The only way for a male to be affected is to inherit the mutant allele from his carrier mother. The chance of this is 1/2, thus Paffected male = 1/2 . A female will be affected if she inherits mutant alleles from both parents. The female in this scenario will always inherit a mutant allele from her father. The chance that she inherits a mutant allele from her carrier mother is 1/2. Thus, Paffected female = 1/2. The overall probability of an affected child is: (½ x ½) + (½ x ½) = ½ You should be able to demonstrate that the Punnett square approach yields the same answer. Problem IV What are the possible modes of inheritance for each pedigree shown below? There may be more than one answer for each pedigree. Please justify each answer. Figure 11 Answers to Problem IV IVA. This pedigree could be: Autosomal dominant (Aa x aa mating) Autosomal recessive (aa x Aa mating) Xlinked recessive (X a Y x X A X a mating) IVB. This pedigree could be: Autosomal dominant (Aa x aa mating) Autosomal recessive (aa x Aa mating) Xlinked recessive (X a Y x X A X a mating) Xlinked dominant (X A Y x X a X a mating) IVC. This pedigree could be: Autosomal dominant (Aa x Aa mating) IVD. This pedigree could be: Autosomal recessive (Aa x Aa mating) IVE. This pedigree could be: Xlinked recessive (X A Y x X A X a mating) Autosomal recessive (Aa x Aa mating) IVF. This pedigree could be: Xlinked recessive (X A Y x X A X a mating) Autosomal recessive (Aa x Aa mating) Table 1 Example Diseases Discussed In Lecture DISEASE Autosomal Dominant HUNTINGTON DISEASE Clinical Case Study #22 MYOTONIC DYSTROPHY CLINICAL FEATURES Note: Key aspects of phenotypic expression or inheritance features are bolded Progressive loss of brain neurons, dementia, loss of motor control Affects 1/20,000 persons of European descent Late onset, typically between 3040 years, but may be earlier (See lecture on unstable trinucleotide repeats.) Facial weakness Cataracts Progressive muscular weakness Variable onset Variable expressivity NEUROFIBROMATOSIS TYPE I Cafeaulait spots (hyperpigmented skin) (NFI) Lisch nodules (benign growths on the iris) Clinical Case Study #29 Peripheral nerve tumors Variable expressivity High mutation rate FAMILIAL HYPERCHOLESTEROLEMIA, Arteriosclerosis, xanthomas Heterozygotes: Increased LDL coronary heart disease in middle age Homozygotes: childhood coronary heart disease Clinical Case Study #14 MARFAN SYNDROME (Connective tissue disorder) Clinical Case Study #26 ACHONDROPLASIA Clinical Case Study #1 Autosomal Recessive CYSTIC FIBROSIS Clinical Case Study #10 TAYSACHS DISEASE Clinical Case Study #38 SICKLE CELL ANEMIA Clinical Case Study #37 XLinked Recessive HEMOPHILIA A Clinical Case Study #18 DUCHENNE MUSCULAR DYSTROPHY Clinical case Study #12 Tall stature with long limbs Narrow facies with high, narrow palate Dislocated lenses & myopia Cardiac manifestations, i.e., aortic aneurysm Variable expressivity Pleiotropy Shortlimbed dwarfism Megalocephaly Lordosis & Kyphosis 80% new mutations Increased mutations with increasing paternal age Chronic, progressive pulmonary disease Pancreatic endocrine insufficiency Elevated sweat chloride Higher frequency in European Caucasians Progressive neurological abnormalities Retinal cherryred spot Higher frequency in the Ashkenazi Jewish and French Canadian populations Reduced serum hexosaminidase A Usually fatal in early childhood Failure to thrive Chronic anemia Vasoocclusive crisis (pain) Increased risk for infection Higher frequency in those of African descent Heterozygote advantage Coagulation disorder Prolonged bleeding Easy bruising Hemorrhage Various mutations & very heterogeneous Progressive muscle weakness Death typically in 2nd or 3rd decade 30% cases due to new mutation Allelic heterogeneity (Becker MD) Xlinked Dominant VITAMIN D RESISTANT RICKETS 1 Rickets Short stature LowSerum phosphate Less severe in heterozygous females The Clinical Case Study number refers to the section in the textbook called "Clinical Case Studies Illustrating Genetic Principles." Use these to learn more about the specific genetic condition.