* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 16
Survey
Document related concepts
Fatty acid synthesis wikipedia , lookup
Cryobiology wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Transcript
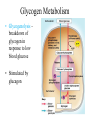
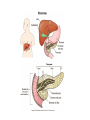
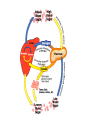
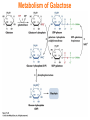
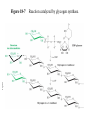
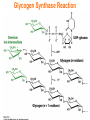
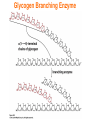
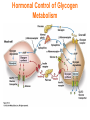
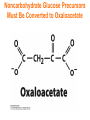
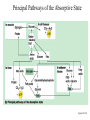
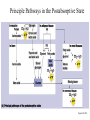
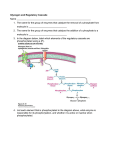
Chapter 16 Glycogen Metabolism and Gluconeogenesis Glycogen Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals and fungi. The polysaccharide structure represents the main storage form of glucose in the body. Glycogen In humans, glycogen is made and stored primarily in the cells of the liver and the muscles hydrated with three or four parts of water. Glycogen functions as the secondary long-term energy storage, with the primary energy stores being fats held in adipose tissue. Muscle glycogen is converted into glucose by muscle cells, and liver glycogen converts to glucose for use throughout the body including the central nervous system. Overview of Glucose Metabolism Glycogen Metabolism Gluconeogenesis • Forms glucose from non-CBH molecules. • In the liver. • Protects the body, especially the brain, from the damaging effects of hypoglycemia by ensuring ATP synthesis can continue. • Stimulated by insulin Glycogen Metabolism • Glycogenolysis – breakdown of glycogen in response to low blood glucose • Stimulated by glucagon Pancreas • Endocrine & exocrine – posterior & slightly inferior to stomach Exocrine function – 98% – production of digestive enzymes by acinar cells Endocrine function – Islets of Langerhans; Pancreas 3 main types of cells α–- produce glucagon β – produce insulin Δ – produce somatostatin Pancreatic Hormones • Insulin Target – general Effect – ↓ blood glucose & ↑diffusion of glucose into cells [not kidney, liver & brain],↑glycogenesis, ↑ uptake of amino acids & peptide formation (↓ gluconeogenesis), ↑ glucose change to fat & ↑ cellular respiration. ↓ glycogenolysis. Pancreatic Hormones Regulation –blood levels of glucose, amino acids & fatty a’s. Pancreatic Hormones • Glucagon Target – liver Effect: ↑blood glucose levels by stimulating gluconeogenesis & glycogenolysis. Regulation – blood glucose levels, ANS & Insulin Structure of Glycogen Glycogen biosynthesis Most important storage form of sugar in animals Glycogen - highly branched (1 per 10) polymer of glucose with (1,4) backbone and (1,6) branch points. More branched than starch so more free ends. Average molecular weight -several million in liver, muscle. 1/3 in liver (more concentrated but less overall mass (5-8%)), 2/3 in muscle (1%). Not found in brain - brain requires free glucose (120 g/ day) supplied in diet or from breakdown of glycogen in the liver. Glucose levels regulated by several key hormones - insulin, glucagon. Glycogen biosynthesis 3 enzymes catalyze the steps involved in glycogen synthesis: UDP-glucose pyrophosphorylase Glycogen synthase Glycogen branching enzyme Glycogen biosynthesis MgATP Glucose HK MgADP [G-1,6-P2] G-6-P F-6-P phosphoglucomutase PGI The hydrolysis of pyrophosphate to inorganic phosphate is highly exergonic and is catalyzed by inorganic pyrophosphatase G-1-P UTP G-1-P PPase PPi UDP-Glucose Pyrophosphorylase 2Pi Metabolism of Galactose Glycogen synthase In this step, the glucosyl unit of UDP-glucose (UDPG) is transferred to the C4-OH group of one of glycogen’s nonreducing ends to form an (1,4) glycosidic bond. Involves an oxonium ion intermediate (half-chair intermediate) Each molecule of G1P added to glycogen regenerated needs one molecule of UTP hydrolyzed to UDP and Pi. UTP is replenished by nucleoside diphosphate kinase UDP + ATP UTP + ADP Figure 18-7 Page 633 O Reaction catalyzed by glycogen synthase. Glycogen synthase All carbohydrate biosynthesis occurs via UDP-sugars Can only extend an already (1,4) linked glucan change. First step is mediated by glycogenin, Glycogenin is an enzyme involved in converting glucose to glycogen. It acts as a primer, by polymerizing the first few glucose molecules, after which other enzymes take over. The protein dissociates after glycogen reaches a minimum size. Glycogen branching Catalyzed by amylo (1,41,6)-transglycosylase (branching enzyme) Rules for branching: Includes non-reducing end Each transferred segment must be at least 11 residues. Each new branch point at least 4 residues away from other branch points. Each new point takes a more exteriorly residue and moves it interiorly Page 634 The branching of glycogen. Schematic two-dimensional cross-sectional view of glycogen: A core protein of glycogenin is surrounded by branches of glucose units. The entire globular granule may contain around 30,000 glucose units. Glycogen Breakdown (glycogenolysis) Requires 3 enzymes: 1. Glycogen phosphorylase (phosphorylase) catalyzes glycogen phosphorylysis (bond cleavage by the substitution of a phosphate group) and yields glucose-1phosphate (G1P) 2. Glycogen debranching enzyme removes glycogen’s branches, allowing glycogen phosphorylase to complete it’s reactions. 92% of glycogen’s glucose residues are converted to G1P and 8% to glucose. 3. Phosphoglucomutase converts G1P to G6P-can either go through glycolysis (muscle cells) or converted to glucose (liver). Process Diagram: Phosphoglucomutase Mechanism Opposing Glycogen Pathways: Synthesis & Degradation Glycogen Storage Diseases Glycogen storage diseases Glycogen storage disease type I (also known as GSDI or von Gierke disease) is an inherited disorder caused by the buildup of glycogen in the body's cells. The accumulation of glycogen in certain organs and tissues, especially the liver, kidneys, and small intestines, impairs their ability to function normally. Glycogen storage diseases Signs and symptoms of this condition typically appear around the age of 3 or 4 months, when babies start to sleep through the night and do not eat as frequently as newborns. Affected infants may have low blood sugar (hypoglycemia), which can lead to seizures. They can also have a buildup of lactic acid in the body (lactic acidosis), high blood levels of a waste product called uric acid (hyperuricemia), and excess amounts of fats in the blood (hyperlipidemia). As they get older, children with GSDI have thin arms and legs and short stature. An enlarged liver may give the appearance of a protruding abdomen. The kidneys may also be enlarged. Affected individuals may also have diarrhea and deposits of cholesterol in the skin (xanthomas). Glycogen Synthase Reaction Glycogen Branching Enzyme Hormonal Control of Glycogen Metabolism Regulation of Phosphoprotein Phosphatase-1 in Muscle Noncarbohydrate Glucose Precursors Must Be Converted to Oxaloacetate Glycolysis & Gluconeogenesis Pathways Pyruvate Conversion to PEP Absorptive and Postabsorptive States • Metabolic controls balance blood concentrations of nutrients between two states: – Absorptive • The time during & shortly after nutrient intake Absorptive and Postabsorptive States – Postabsorptive • The time when the GI tract is empty. • Energy sources are supplied by the breakdown of body reserves. Absorptive State • Ingested nutrients enter blood and lymphatic system --> hepatic portal system to liver • Lasts about 4 hours after completing a meal Events: Absorptive State • Glucose – Glucose uptake by liver converted to triglycerides and glycogen (10%) – Adipose tissues store fat take up blood glucose to triglycerides (40%) – Muscles take up glucose and store as glycogen (50%) Absorptive State Events: • Amino Acids liver Kreb's cycle or gluconeogenesis or protein synthesis • Lipids most packaged VLDL lipoproteins and are carried to adipose. • Hormones -mostly, insulin [hypoglycemic hormone] Absorptive State Figure 24.18a Principal Pathways of the Absorptive State Figure 24.18b Postabsorptive State • Need to maintain normal blood glucose level [90-100mg/100mL] • Very important for nervous system - can only use glucose for energy. Postabsorptive State EVENTS: • Liver glycogen is converted to glucose - lasts about 4 hrs. • Muscle glycogen is converted to lactic acid glucose in liver • Adipose breaks triglycerides to glycerol glucose Postabsorptive State • Muscle protein aa converted by liver into glucose [gluconeogenesis] • Hormone – glucagon; Neural Control – ANS via epinephrine Postabsorptive State Figure 24.20a Principle Pathways in the Postabsorptive State Figure 24.20b General Adaptation Syndrome [GAS] • Response to prolonged, extreme or unusual stress • Stressor – and disturbance – temperature, toxins, poisons, heavy bleeding, emotional upheaval General Adaptation Syndrome [GAS] • GAS – 3 stages 1 – Alarm Reaction = Fight or flight Hypothalamus stimulates ANS & adrenal medulla epinephrine/ norepinephrine; Short lived Consumes glycogen stores General Adaptation Syndrome [GAS] 2 – Resistance Stage [long-term] Provide alternative fuels when glycogen has been depleted. Dominated by cortisol.