* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction to Atoms
Survey
Document related concepts
Transcript
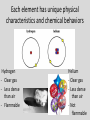
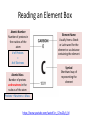
Introduction to Atoms What is matter? • Matter is anything you can touch. • Includes – Solids – Liquids – Gases Yes, you can touch a gas, you can feel the air molecules hitting your face when a fan is on. Matter is made of smaller particles How was this painted? Small particles combine to make the larger object How was this made? www.chrisjordan.com What do these pieces of art have to do with matter? Like the art, matter is made of small particles • It's also like a picture stored in a computer... • From a distance, it looks continuous, but if you blow it up, • You can see the individual pixels that it is made from. Legos • It's also like building things out of Legos... • You can take small, individual pieces and put them together to make something bigger. These small particles are called atoms Atoms are tiny https://www.youtube.com/watch?v=yQP4UJhNn0I https://www.youtube.com/watch?v=oSCX78-8-q0 Atom • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus How do we know about atoms? • https://www.youtube.com/watch?v=xazQRcS CRaY Ted History of Atoms (5:30mins) • https://www.youtube.com/watch?v=thnDxFdk zZs Crash Course Chemistry #37 (9 min) An atom is the smallest unit of an element • There are 118 known elements • An element is a substance that cannot be broken down into simpler substances. Each element has unique physical characteristics and chemical behaviors Each element has unique physical characteristics and chemical behaviors Hydrogen - Clear gas - Less dense than air - Flammable Helium - Clear gas - Less dense than air - Not flammable An atom is the smallest unit of an element that has all the physical characteristics and chemical behaviors of that element. So how do I know what element I have? ? The number of protons in an atom’s nucleus tells us what element it is. No two elements have the same number of protons. So how do I know how many protons are in an element? Reading the Periodic Table • The atomic number tells us how many protons are in the nucleus. • • • • How many protons are in aluminum? 13 Nitrogen? 7 Sulfur? 16 Carbon? 6 The periodic table is arranged by atomic number- smallest to largest The element block will also include a symbol for the element • The symbol is a short hand way of writing the element’s name • Some elements have a single letter for their symbol • The first letter is ALWAYS capitalized • If there is a second letter, it is ALWAYS lower case Element symbols and some unusual names https://www.youtube.com/watch?v=p90Ug56GkcE Review 1. Everything on Earth is made of ________ (anything you can touch) - matter Review 2. Matter is made of small particles called ____ -Atoms Review 3. Atoms are made of three smaller particles called _____ -protons, neutron, and electrons Review 4. An atom is the smallest unit of an ________ -Element Review 5. Each element is different, physically and chemically, because each element has a different number of ______ in its nucleus - protons Review 6. The _______ _________ tells us how many protons are in the nucleus. -Atomic number Review 7. The element can be represented by a symbol. The first letter is always ______ and the second letter is always _______ ________ -capitalized -lower case Homework-Element Gossip • Use the atomic number given to find the element. • Write the element symbol on the blanks (1st letter of the symbol is capitalized, 2nd (if present) is lower case. • Then write the full name of each element. • Periodic tables can be found in your agenda book or in the textbook inside covers. Reading an Element Box Atomic Number Number of protons in the nucleus of the atom # of Protons = # of Electrons Atomic Mass Number of protons and neutrons in the nucleus of the atom Element Name Usually from a Greek or Latin word for the element or a substance containing the element Symbol Shorthand way of representing the element Protons + Neutrons = Mass https://www.youtube.com/watch?v=_S7ov25y3_M 1. What is the symbol? C 2. What is the element? Carbon 3. What is the atomic number? 6 4. What does atomic number tell us? Number of protons in nucleus Protons = electrons 5. What is the atomic mass? 12 (round to nearest whole number) 6. What does the atomic mass tell us? Protons + neutrons in the nucleus Practice On the back • Draw an atom of Lithium – How many protons, neutrons, and elections? – Where do they go? – 3 protons – 4 neutrons – 3 electrons On the back • Draw an atom of Oxygen – How many protons, neutrons, and elections? – Where do they go? – 8 protons – 8 neutrons – 8 electrons On the back • Draw an atom of Iron – How many protons, neutrons, and elections? – Where do they go? – 26 protons – 30 neutrons – 26 electrons Homework On https://www.youtube.com/watch?v=_lNF3_30l UE
















































![Atomic Structure [PowerPoint]](http://s1.studyres.com/store/data/000122096_1-1d100da6540d2f26db122fc51f672fe5-150x150.png)










