* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Reactions of Osmium(VIII) in Hydroxide
Woodward–Hoffmann rules wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Acid–base reaction wikipedia , lookup
Electrochemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Industrial catalysts wikipedia , lookup
Ene reaction wikipedia , lookup
Rate equation wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
The Reactions of Osmium(VIII) in Hydroxide Medium
By
Theodor Earl Geswindt
Submitted in fulfilment of the requirements for the degree
of Magister Scientiae at the Nelson Mandela Metropolitan
University
January 2009
Supervisor: Prof. H. E. Rohwer
I
ACKNOWLEDGEMENTS
I am deeply grateful to my supervisor, Professor H. E. Rohwer, for his encouragement,
advice, guidance and thoughtful discussions.
I would also like to express my sincere thanks to:
Dr Willem Gerber for his time, effort and knowledgeable insight.
Dr Eric Hosten and Henk Schalekamp – my poor proof-readers.
Anglo Platinum Research Centre, the Nelson Mandela Metropolitan University and
the Inorganic Chemistry Department for financial assistance.
My parents for all their love and support.
Evron for all her love and endless moral support.
God for making all things possible.
II
TABLE OF CONTENTS
Acknowledgements
I
Table of Contents
II
List of Figures
VI
List of Tables
XI
Abbreviations
XII
Summary
XIII
CHAPTER 1
Introduction
1.1 History of osmium
1
1.2 Extraction of osmium
2
1.3 Applications of osmium
4
1.4 General coordination chemistry
5
1.4.1 The Common oxidation states of osmium
6
1.4.2 Coordination numbers
7
1.4.3 Coordinating ligands
8
1.5 Aims and objectives
9
CHAPTER 2
Experimental
2.1 Apparatus
10
2.1.1 UV-Vis spectrophotometric recordings
10
2.1.2 Mole ratio titrations
11
2.1.3 Potentiometric titrations
12
2.1.4 pH measurements
12
2.1.5 Potentiometric measurements
12
2.1.6 Preparation of solutions
12
2.2 Computer hardware and software
13
2.3 Reagents utilised
14
2.4 Standardisation methods
16
III
2.4.1 Standardisation of acids
16
2.4.2 Preparation of a standard sodium hydroxide solution
16
2.5 Preparation and storage of osmium tetroxide
16
2.5.1 Introduction
16
2.5.2 Preparation procedure
17
2.5.3 Preparation of aqueous osmium tetroxide
19
2.6 Preparation of potassium osmate
19
2.7 Determination of osmium concentration – the thiourea colourimetric
method
20
2.7.1 Introduction
20
2.7.2 The effect of varying thiourea concentration on the formation of the
[Os(NH2CSNH2)6]3+ species
22
2.7.2.1 Literature review
22
2.7.2.2 Experimental procedures
22
2.7.2.3 Results and discussion
23
2.7.3 The effect of varying hydrochloric acid concentration on the
formation of the [Os(NH2CSNH2)6]3+ species
25
2.7.3.1 Literature review
25
2.7.3.2 Experimental procedures
26
2.7.3.3 Results and discussion
28
2.7.4 The osmium – thiourea calibration curve
36
2.7.4.1 Literature review
36
2.7.4.2 Experimental procedures
36
2.7.4.3 Results and discussion
37
CHAPTER 3
The alcohol reduction of osmium(VIII) in hydroxide medium
3.1 Introduction
37
3.2 Isosbestic points
46
3.3 The stability of osmium(VIII) in a 2M hydroxide matrix
47
3.3.1 Literature review
47
3.3.2 Experimental procedures
50
IV
3.3.3 Results and discussion
51
3.4 The reduction of osmium tetroxide by aliphatic alcohols in a 2M
hydroxide matrix
55
3.4.1 Literature review
55
3.4.2 Experimental procedures
56
3.4.3 Results and discussion
59
3.5 The geometrical analysis of kinetic data using Mauser diagrams
67
3.5.1 Literature review
67
3.5.2 Experimental procedures
71
3.5.3 Development of computational software for data analysis
71
3.5.4 Results and discussion
73
3.6 The osmium(VIII) – alcohol kinetic model
77
3.6.1 Literature review
77
3.6.2 Experimental procedures
78
3.6.3 Computational software utilised for kinetic modelling
79
3.6.4 Results and discussion
81
CHAPTER 4
The osmium(VIII)-osmium(VI) equilibrium reaction
4.1 Introduction
93
4.2 The stability of osmium(VI) in a 2M hydroxide matrix
94
4.2.1 Literature review
94
4.2.2 Experimental procedures
94
4.2.3 Results and discussion
96
4.3 The osmium(VIII) – osmium(VI) reaction
4.3.1 Literature review
100
100
4.3.1.1 Job’s method of continuous variation
100
4.3.1.2 Mole ratio titrations
104
4.3.2 Experimental procedures
104
4.3.2.1 Job’s method of continuous variation
104
4.3.2.2 Mole ratio titrations
105
4.3.3 Computer software used for simulating mole ratio titrations
106
V
4.3.4 Results and discussion
107
4.3.4.1 Job’s method of continuous variation
107
4.3.4.2 Mole ratio titrations
113
4.4 Conclusion
116
CHAPTER 5
Conclusion
5.1 Determination of osmium concentration
117
5.2 The osmium(VIII) – alcohol reaction
117
5.3 The osmium(VIII) – osmium(VI) complexation
120
APPENDIX
Development of the program GP2
A.1 Introduction
123
A.2 Listing of the program GP2
126
REFERENCES
127
VI
LIST OF FIGURES
Chapter 1
Figure 1.1:
Page
Extraction of four of the platinum group metals from platinum ore concentrates; a
simplified overall scheme. The path highlighted in red indicates the reaction
investigated during this study.
3
Chapter 2
Figure 2.1:
Illustration of the experimental system employed to record UV-Vis spectra at
constant temperatures
Figure 2.2:
11
Illustration of the experimental setup used during the preparation of a pure OsO4
solution
Figure 2.3:
18
UV-Vis spectra illustrating the formation of the [Os(NH2CSNH2)6]
3+
species as a
function of thiourea concentration. The direction of the solid arrows indicates
increasing thiourea concentration. [HCl] = 5.091 mol/L;
-4
[Osmium] = 1.051 × 10 mol/L; solutions were equilibrated for 8 days at 25°C
Figure 2.4:
23
The change in absorbance at 490 nm as a function of thiourea concentration,
indicating the large excess of thiourea required for the complete conversion of
2-
3+
[OsCl6] to the [Os(NH2CSNH2)6]
species. The ratio of thiourea:osmium should
2-
3+
be at least 4300:1 in order for full conversion of [OsCl6] to [Os(NH2CSNH2)6] .
Figure 2.5:
UV-Vis spectra of the [OsCl6] reduction by thiourea as a function of HCl
-4
concentration. [Thiourea] = 0.657 M; [Osmium] = 1.871 × 10 M
Figure 2.6:
28
The change in absorbance at selected wavelengths as a function of HCl
-4
concentration. [Thiourea] = 0.657 M; [Osmium] = 1.871 × 10 M
Figure 2.7:
24
2-
29
2-
UV-Vis spectra depicting the reduction of [OsCl6] by thiourea as a function of HCl
concentration at an ionic strength of 6 M. The solid arrows indicate the direction of
increasing [HCl]. The [HCl] ranges from 0.750 M to 5.250 M;
-4
2-
[Osmium] = 2.103 × 10 M. The spectrum of pure [OsCl6] is included for
comparison.
Figure 2.8:
30
-
The absorbance at selected wavelengths as a function of the mole fraction Cl
-
ClO4 ]
Figure 2.9:
The absorbance at selected wavelengths as a function of [mole Cl / mole
Figure 2.10:
UV-Vis spectra depicting the reduction of osmium tetroxide by thiourea as a
31
31
function of HCl concentration at an ionic strength of 6 M. [Thiourea] = 0.657 M;
-5
[Osmium] = 6.554 × 10 M; [HCl] ranges from 0.500 M to 6.000 M
Figure 2.11:
33
The change in absorbance at selected wavelengths as a function of HCl
concentration
33
VII
Figure 2.12:
UV-Vis spectra of the standard ammonium hexachloroosmium(IV)-thiourea
solutions recorded after 8 days at 25°C. [Thiourea] = 0.657 M; [HCl] = 5.091 M; the
respective osmium concentrations are noted in the figure.
Figure 2.13:
37
The calibration curve obtained through the thiourea colourimetric method.
Calibration curve constructed from the absorbance data at 490 nm
38
Figure 3.1:
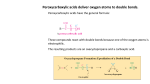
The generally accepted mechanism for the oxidation of alkenes to cis-diols
40
Figure 3.2:
(a) syn- and (b) anti- dimeric monoesters, (c) monomeric diester
41
Figure 3.3:
Proposed pathways (a and b) for the reaction of organic reductant (R) with osmium
Chapter 3
tetroxide
Figure 3.4:
42
The mechanism proposed by Sharpless et al involving nucleophillic attack by the
C – C double bond on the electropositive osmium
43
Figure 3.5:
Proposed reaction scheme for the oxidation of alcohols by osmium tetroxide
43
Figure 3.6:
Mechanism of alcohol oxidation by chromic acid (Westheimer’s “ester mechanism”)
45
Figure 3.7:
The UV-Vis spectra of OsO4 in both CCl4 and water, obtained during this study.
The spectrum of gaseous OsO4 is included for comparison
48
Figure 3.8:
Species distribution diagram for OsO4 as a function of pH
49
Figure 3.9:
The change in the UV-Vis spectrum of osmium(VIII) in 2 mol/L NaOH as a function
of time, from t = 0 hour to t = 625 hours. The spectra change in the direction of the
-4
solid arrows over time. [Osmium] = 1.305 × 10 mol/L
Figure 3.10:
51
Progress curve depicting the rate of change of absorbance at 370 nm.
-4
[Osmium] = 1.305×10 mol/L; [NaOH] = 2 mol/L
Figure 3.11:
52
-4
The spectra isolated during the reduction of 1.305 × 10 mol/L osmium(VIII) in
-4
2 mol/L NaOH. The spectrum of a 1.305 × 10 mol/L osmium(VI) solution in
2 mol/L NaOH is included for comparison.
Figure 3.12:
54
The change in the osmium(VIII) optical spectrum as a function of time, from t = 0 to
-3
t = 986 minutes, upon addition of 1.00 × 10 mol/L methanol. The spectra denoted
by A, B, and C respectively refer to the spectra recorded at t = 0, t = 34 and t = 986
minutes. The solid arrow indicates the direction of absorbance change with time.
The solid and dashed lines indicate the occurrence of two isosbestic points.
-4
-
[Osmium] = 1.305 × 10 mol/L; [OH ] = 2 mol/L
Figure 3.13:
59
Illustration of the isosbestic points formed during the reduction of osmium tetroxide
by methanol in 2 mol/L hydroxide medium. (a) The first transient isosbestic point
occurs at 274 nm as indicated by the dashed line; (b) the second isosbestic point
occurs at 258 nm as indicated by the solid line
Figure 3.14:
60
Progress curves demonstrating the rate of change of the absorbance at 370 nm at
-4
different methanol concentrations. [Osmium] = 2.631 × 10 mol/L;
[NaOH] = 2 mol/L; Methanol concentrations are denoted by the legend.
62
VIII
Figure 3.15:
(a) Spectra isolated at various times during the reduction of osmium(VIII) by
-4
-3
methanol. [Osmium] = 2.631 × 10 mol/L; [Methanol] = 1.00×10 mol/L;
[NaOH] = 2 mol/L. (b) A comparative reduction reaction conducted in the absence
-4
of methanol. [Osmium] = 1.305 × 10 mol/L; [NaOH] = 2 mol/L. In both figures the
times at which these spectra were recorded are denoted in the legend.
Figure 3.16:
63
Progress curves indicating the rate of change of the absorbance at 370 nm for
various methanol concentrations. The progress curve depicting the reaction of
osmium(VIII) with 0 mol/L methanol was superimposed onto the progress curves
for those reactions involving varying methanol concentrations.
-4
[Osmium] = 2.631 × 10 mol/L; [NaOH] = 2 mol/L; methanol concentrations are
denoted by the legend.
Figure 3.17:
64
Progress curves illustrating the change in absorbance at 370 nm as a function of
time for the reaction between osmium tetroxide and varying ethanol
-4
concentrations. [Osmium] = 2.590 × 10 mol/L; [NaOH] = 2 mol/L; ethanol
concentrations are denoted in the legend.
Figure 3.18:
65
Progress curves illustrating the change in absorbance at 370 nm as a function of
time for the reaction between osmium tetroxide and varying propan-1-ol
-4
concentrations. [Osmium] = 2.285 × 10 mol/L; [NaOH] = 2mol/L; propan-1-ol
concentrations are denoted by the legend.
Figure 3.19:
65
Progress curves illustrating the change in absorbance at 370 nm as a function of
time for the reaction between osmium tetroxide and varying butan-1-ol
-4
concentrations. [Osmium] = 2.212 × 10 mol/L; [NaOH] = 2 mol/L; butan-1-ol
concentrations are denoted by the legend.
↔B↔C
Figure 3.20:
Typical 2-dimensional Mauser diagram for the general reaction A
Figure 3.21:
The change in the Osmium (VIII) optical spectrum as a function of time, upon
66
70
-3
addition of 1.00 × 10 mol/L methanol. The spectra denoted by A, B and C
respectively refer to the spectra recorded at t = 0, t = 34 and t = 986 minutes. The
solid arrow indicates the direction of increasing time.
-4
-
[Osmium] = 1.305 × 10 mol/L; [OH ] = 2 mol/L
Figure 3.22:
73
[a] 3D Mauser diagram of A370 vs. A240 vs. A280 (the indices indicate the
wavelengths used). [b] Rotation of part [a]. The curve lies on a single plane, and
is viewed along the edge of this plane. The result is a straight line indicating the
case s = 2.
74
Figure 3.23:
A 2D Mauser diagram constructed from the data presented in Figure 3.21.
75
Figure 3.24:
Molar extinction spectrum for the Os(VII)-Intermediate species, calculated using
the program GP2.
76
IX
Figure 3.25:
Comparison between the theoretical fits obtained for [a] methanol and [b] propan1-ol, based on Model 4. The comparison illustrates the pronounced effect that a
loss of kinetic data has on the theoretical fit. Symbols = Experimental data;
-4
Lines = Theoretical fit. [a] [Osmium] = 2.631 × 10 mol/L;
-3
-4
[Methanol] = 15 × 10 mol/L. [b] [Osmium] = 2.285 × 10 mol/L; [Propan-1-3
ol] = 15 × 10 mol/L.
87
Figure 3.26:
2D Mauser diagram interpreted in terms of the proposed kinetic model, Model 4.
89
Figure 3.27:
The E2 C – H bond cleavage reaction mechanism
90
Figure 3.28:
The hydride transfer reaction mechanism – from the associative reaction of the
primary alcohol molecule with the osmium(VIII) centre, leading to the formation of
the osmate ion and the aldehyde.
91
Chapter 4
Figure 4.1:
2-
The change in the UV-Vis spectrum of [OsO2(OH)4] upon exposure to an oxygen
atmosphere, as a function of time. The dashed arrows respectively depict the
spectra recorded at t = 0 min and t = 2868 min. The solid arrow indicates the
-4
direction of increasing time. [Osmium] = 5.278 × 10 mol/L; [NaOH] = 2 mol/L
Figure 4.2:
The change in absorbance at 300 and 350 nm as a function of time under oxygen
-4
atmosphere. [Osmium] = 5.278 × 10 mol/L; [NaOH] = 2 mol/L
Figure 4.3:
97
2-
The change in the UV-Vis spectrum of [OsO2(OH)4] upon exposure to a nitrogen
-4
atmosphere. [Osmium] = 4.578 × 10 mol/L; [NaOH] = 2 mol/L
Figure 4.4:
98
The change in absorbance at 300 and 350 nm as a function of time under nitrogen
-4
atmosphere. [Osmium] = 4.578 × 10 mol/L; [NaOH] = 2 mol/L
Figure 4.5:
96
98
The change in absorbance spectra as a function of increasing [Os(VI)]i / [Os(VIII)]i
ratio at pH 14.3. The spectra denoted [1], [14] and [29] corresponds to the
[Os(VI)]i / [Os(VIII)]i ratios 0.03, 0.94 and 30.00 respectively.
-4
[Os(VI)] + [Os(VIII)] = 3.485 × 10 mol/L
Figure 4.6:
107
-4
The UV-Vis spectra of 3.485 × 10 mol/L OsO4 in a 2 mol/L NaOH matrix;
-4
3.485 × 10 mol/L potassium osmate in a 2 mol/L NaOH matrix; the experimentally
-4
observed spectrum obtained from the reaction between 1.799 × 10 mol/L
-4
osmium(VIII) with 1.686 × 10 mol/L potassium osmate in a 2 mol/L NaOH matrix;
the theoretically calculated addition spectrum between osmium(VIII) and potassium
osmate; and a comparison of the intermediate species’ spectrum obtained by
reacting osmium(VIII) with methanol in a 2 mol/L NaOH matrix.
Figure 4.7:
108
Non-equimolar Job diagram illustrating complex formation between osmium(VIII)
and osmium(VI) in a 2 mol/L NaOH matrix.
-4
-4
[Os(VI)] + [Os(VIII)] = [1] 3.485 × 10 mol/L; [2] 7.000 × 10 mol/L
109
X
Figure 4.8:
Job diagram depicting the complex formation between osmium(VIII) and
osmium(VI) in a 2 mol/L NaOH matrix. The theoretical fits were simulated on a 1:1
-4
complexation model. [Os(VI)] + [Os(VIII)] = 3.485 × 10 mol/L.
Symbols = experimental data; Lines = calculated fits.
Figure 4.9:
110
Correcting a Job plot for the absorbance of the reacting components, as reported
in literature. Lines [2] and [3] is subtracted from plot 1 to obtain plot 4. Experimental
data are from an osmium(VIII) – osmium(VI) Job plot at pH 14.3.
-4
[Os(VI)] + [Os(VIII)] = 3.485 × 10 mol/L
Figure 4.10:
111
Absorbance curves from several osmium(VIII) vs. osmium(VI) mole ratio titrations
in a 2 mol/L NaOH matrix. Linear regressions were drawn though the linear regions
of the curves to obtain the point of intersect. The initial osmium(VIII) concentration
is denoted in the legend.
Figure 4.11:
113
Volume corrected absorbance curves from osmium(VIII) vs. osmium(VI) mole ratio
titrations in a 2 mol/L NaOH matrix. The calculated curves were simulated on a 1:1
complexation model. The initial osmium(VIII) concentrations are denoted in the
figure. Symbols = experimental data; Lines = calculated fits
Figure 4.12:
Species distribution curves of an osmium(VIII) vs. osmium(VI) titration in a 2 mol/L
-4
Figure 4.13:
114
NaOH matrix. [Os(VIII)] = 2.327 × 10 mol/L
115
The formation of the dimeric osmium(VII) species
116
Program interface after data selection
124
Appendix
Figure A 1:
XI
LIST OF TABLES
Chapter 1
Table 1.1:
Page
The more unusual coordination numbers for osmium
7
Chapter 2
Table 2.1:
Computer platform used for kinetic and equilibrium calculations
13
Table 2.2:
Reagents utilised during this study
14
Chapter 3
Table 3.1:
The second order rate constants for the oxidation of several alcohols by
-4
osmium(VIII) in a hydroxide matrix; [Osmium] = 3.16 × 10 mol/L,
-
[OH ] = 0.05 mol/L, Temp = 305 K
Table 3.2:
Reactant volumes and concentrations for the reaction of osmium tetroxide with
methanol in a 2 mol/L hydroxide matrix
Table 3.3:
58
Calculated rate constants and molar extinction coefficients for the reduction of
osmium(VIII) by several primary alcohols at pH 14.3, based on Model 4
Table 3.4:
44
87
Comparison between the molar extinction coefficient (at various wavelengths) of
the Os2(VII) species calculated from a least squares [LS] method and Mauser
diagrams [MD]
88
Chapter 4
Table 4.1:
The molar extinction coefficients and averaged equilibrium constant calculated at
various wavelengths through Job’s method of continuous variation.
Table 4.2:
112
Comparison of the molar extinction coefficients and equilibrium constant obtained
from mole ratio studies and Job diagrams at 400 nm
115
Chapter 5
Table 5.1:
Comparison of the equilibrium constant and molar extinction coefficients calculated
through several computational methods. MD = Mauser diagrams; LS = Least
square analysis; JD = Job diagrams; MR = Mole ratio titrations
121
XII
ABBREVIATIONS
ETAAS
electrothermal atomic absorption spectroscopy
HCl
hydrochloric acid
HClO4
perchloric acid
ICP-MS
inductively coupled plasma mass spectrometry
m/v
mass per volume
mL
millilitre
M
mol per litre
mol/L
mol per litre
NaOH
sodium hydroxide
UV-Vis
ultraviolet – visible
v/v
volume per volume
XIII
SUMMARY
Spectrophotometric techniques were used to elucidate the discrepancies surrounding
the reduction of osmium tetroxide by several primary alcohols in a hydroxide matrix. In
contrast to the documented literature, this reaction was observed to occur in two
consecutive reaction steps. Geometrical and computational analysis of kinetic data
revealed that the reaction proceeds by the following reaction model:
Os(VIII) + RCH2OH
Os(VIII) + Os(VI)
k1
k+2
k-2
Os(VI) + RCHO
Os2(VII)
The conditional rate constants and molar extinction coefficients were calculated using
custom written software. A hydride transfer mechanism, coupled with the synchronous
removal of the hydroxyl proton of the alcohol, was postulated.
The complexation between osmium(VIII) and osmium(VI) was investigated. Mole ratio
titrations and mole fraction plots show that at pH 14.3 a 1:1 complexation occurs
between osmium(VIII) and osmium(VI). The equilibrium constants and molar extinction
coefficients calculated by these methods were found to be consistent with the
parameters obtained from the reduction of osmium tetroxide by primary alcohols at pH
14.3.
The formation of a mixed oxidation state dimeric osmium complex (denoted
Os2(VII)) has been proposed.
Key words: Spectrophotometric techniques, osmium tetroxide, osmium(VIII), primary
alcohols, osmium(VI).
1
CHAPTER 1
Introduction
1.1
History of Osmium
Osmium, so named by its discoverer Smithson Tennant, from the characteristic odour of
its tetroxide (derived from the Greek word osme – smell, odour). Tennant, who was
Professor of Chemistry at the University of Cambridge, discovered the new element in
1803 and announced it in 1804. He had heated (to red heat with caustic soda) the
insoluble black residues which were left after the digestion of native platinum by aqua
regia, and dissolved the resulting mass in water. The yellow filtrate was acidified, which
led to the evolution of a white, volatile oxide, of which Tennant wrote[1]:
“It stains the skin of a dark colour which cannot be effaced… (it has) a pungent
and penetrating smell… from the extrication of a very volatile metal oxide… this
smell is of its most distinguishing characters, I should on that account incline to
call the metal Osmium”
In this latter statement, it is interesting to note that Tennant had earlier proposed to call
the element ptène (from ptenos – meaning volatile), but was persuaded to abandon this
idea [1].
The “volatile metal oxide” Tennant referred to was osmium tetroxide, which is now
known to have highly toxic effects, as documented by Brunot [2], who exposed himself to
the toxic vapours in order to ascertain its toxicity.
Upon exposure to the osmium
tetroxide vapour he noticed a metallic taste in his mouth and found that smoking was
unpleasant; after 30 minutes his eyes were smarting and tearing; after three hours his
chest was constricted and he had difficulty breathing and on going outside he noticed
large haloes around street lights. Subsequent research into the toxicity of osmium
tetroxide revealed that the unpleasant side effects experienced by Brunot were a result
of the reduction of osmium tetroxide onto the eyes, skin, and mucosa of the airways.
2
Concentrations in the air as low as 10-7 g.m-3 can cause lung congestion, skin and
severe eye damage[3].
Due to its toxicity, every care must be taken when working with osmium in all its forms,
since it can be oxidised by atmospheric oxygen to the tetroxide.
1.2
Extraction of Osmium
The modern method for extracting the element does not differ greatly from the
Tennant’s original procedure[1]. Platinum bearing concentrates are extracted with aqua
regia and the insoluble portion heated in an oxidising flux such as sodium peroxide.
The residue is extracted with water. The insoluble fraction is treated for rhodium and
iridium while the soluble fraction contains perosmate, [OsO4(OH)2]2-, and ruthenate,
[RuO4]2-, ions. At this stage, the osmium is removed with nitric acid, producing the
volatile tetroxide. Alcohol can be added to the alkaline solution, which precipitates the
ruthenium as the hydrated dioxide and reduces the perosmate to the soluble violet
potassium osmate, K2[OsO2(OH)4]. This reaction route is highlighted in Figure 1.1. The
potassium osmate can be precipitated by the addition of concentrated potassium
hydroxide, which can be acidified to produce the volatile osmium tetroxide. Potassium
osmate can also be treated with an alcohol-hydrochloric acid-ammonium chloride
mixture to produce the ammonium hexachloroosmate(IV). The latter species may then
be heated in an inert atmosphere in a graphite vessel to give the pure metal, which
could also be obtained by reduction of osmium tetroxide in hydrogen.
Figure 1.1 depicts a simplified overview of the industrial separation of four platinum
group metals (rhodium, osmium, ruthenium and iridium) from platinum metal
concentrates.
The highlighted path indicates the reaction which would be under
investigation during this study.
3
Insoluble residue from treatment of
platinum metal concentatrates with aqua
reagia is smelted with lead carbonate and
then treated with nitric acid to remove
silver as the nitrate.
For
ores
of
low
rhodium content the
bisulphate step is omitted
Insoluble
residue
Fuse with
NaHSO4 (500°)
Insoluble
Soluble
Fuse with
Na2O2 (500°)
Rh2(SO4)3•aq
Soluble
NaOH
Rh(OH)3•aq
Insoluble residue
of IrO2•aq
[OsO4(OH)2]2- + [RuO4]2-
HCl
KOH
C2H5OH
H3[RhCl6]•aq
Soluble
NaNO2
+
NH4Cl
(NH4)3[Rh(NO2)6]
[OsO2(OH)4]2-
HCl
NH4+
HNO3
(NH4)3[RhCl6]
OsO4
H.COOH
H2 at 1000°
HCl, NH4+
C2H5OH
Rhodium
(NH4)2[OsCl6]
H2 at 1000°
Osmium
HCl,
HNO3,
NH4+
Insoluble
RuO2•aq
HCl
H2[RuCl6]•aq
Cl2
(NH4)2[IrCl6]
H2
at 1000°
Iridium
RuO4
HCl, NH4+
(NH4)3[RuCl6]
H2 at 1000°
Ruthenium
Figure 1.1: Extraction of four of the platinum group metals from platinum ore concentrates; a
[1]
simplified overall scheme . The path highlighted in red indicates the reaction investigated during
this study.
4
1.3
Applications of Osmium
Due to its density (22.587 ± 0.009 g.cm-3), osmium is frequently used in small quantities
in alloys where frictional wear must be minimised. These alloys are typically used in
ballpoint pen tips, fountain pen tips, record player needles, electrical contacts and high
pressure bearings. It is therefore not surprising that osmium is no longer considered
industrially important, considering this list of applications. A less dated application of
osmium is in the platinum/osmium (in a 90:10 ratio) alloy used in implants such as
pacemakers and replacement valves[4]. This alloy is used predominantly due to its
resistance to corrosives, but is difficult and expensive to manufacture.
Osmium
tetroxide, although highly toxic, is still used as a biological fixative, for the preservation
of biological tissue and its delineation for optical and electronic microscopy[5].
The most useful application of osmium tetroxide is its ability to act as a catalyst in
organic oxidation reactions. The most famous of these reactions is the industrially
important cis-hydroxylation of alkenes; in which osmium tetroxide selectively
cis-hydroxylate unsaturated organic compounds.
5
1.4
General Coordination Chemistry
Osmium is considered as the most versatile of all the platinum group metals, even more
so than Rhenium and Ruthenium. This is exhibited through the wide array of oxidation
states (VIII to –II) displayed by its complexes. The major reason for its versatility is
primarily due to the position that osmium assumes within the group of transition metals
in the period table[6]. As a member of the third row of transition metals, the outer 5d
orbitals are fairly exposed, effectively increasing the susceptibility of the electronic
occupancy of the 5d orbitals to the coordinating ligands. In addition, its central position
within the third row transition metals implies the attainment of[6]:
o
the d0 electronic configuration typical of the elements to the left of osmium in the
periodic table
o
the d10 electronic configuration typical of the elements to the right of osmium in
the period table
Osmium can thus be classified as a metal which adopts various oxidation states through
the nature of the coordinating ligands, making the chemistry of osmium unique and
dynamic.
High oxidation state osmium (the VIII, VII and VI oxidation states) is associated with
strong σ- and π-donor ligands such as F-, O2- and N3-, since these ligands tend to form
stable complexes with ions possessing few or no d-electrons. Medium oxidation state
osmium (the V, IV and III oxidation states) is associated with ligands having σ-donating
capabilities such as NH3, halides (F-, Cl-, Br- and I-) and ethylenediamine[6].
Low
oxidation state osmium (the II to -II oxidation states) is associated with ligands having
strong π-acceptor capabilities such as CO and NO+, while ligands with moderate πacceptor capabilities (e.g. CN-) will tend to favour the osmium(II) (d6) oxidation state.
6
1.4.1
The Common Oxidation States of Osmium[6]
Osmium(II)
Osmium(II) is a d6 ion, generally with a spin-paired (t2g6) electron configuration and is
therefore considered as diamagnetic.
Generally, octahedral complexes are formed,
although five- and seven-coordinate complexes are known to exist.
Osmium(II)
complexes are easily oxidised through atmospheric oxygen, although it can be
stabilised through the coordination of mild π-acceptor ligands such as phosphines,
cyano-groups, CO, and aromatic amines.
Osmium(III)
Osmium(III) is a spin-paired d5 ion (t2g5) with an octahedral geometry exhibited by its
complexes. This oxidation state illustrates extensive reactions with σ-donor, π-acceptor
ligands such as N -, O -, S - and P -donor ligands. However, due to the single, unpaired
electron within the t2g sub-shell, osmium(III) is prone to oxidation to the tetravalent
oxidation state or reduction to the divalent oxidation state.
Osmium(IV)
The tetravalent oxidation state of osmium is its most common oxidation state, owing its
stability to the coordination of good σ-donor, π-acceptor ligands to the metal ion. The
osmium(IV) ion is spin-paired when existing in an octahedral milieu. Although the ion
has two unpaired electrons in the t2g sub-shell, its magnetic properties are anomalous
due to the quenching of electron spin by orbital spin. Most of its complexes are anionic
or neutral (e.g. OsCl62-) although a few cationic species does exist (e.g. [Os(diars)2X2]2+,
where X = Cl-, Br- and I-).
Osmium(VI)
The hexavalent oxidation state is frequently associated with σ-donor, π-donor ligands
such as F-, O2- and N3-. The chemistry of these complexes are dominated by the oxospecies (more specifically, the octahedral trans-[OsO2(OH)4]2- species) and the nitridospecies (the octahedral [OsNCl5]2- species).
It has been reported that the nitrido-
species are more readily formed with osmium than with any other metal ion.
7
Osmium(VIII)
The most important complex in this oxidation state is the stable, tetrahedral osmium
tetroxide, OsO4.
However, the fluoro-complex, [OsO3F3]-), and nitrido-complex,
([OsO3N]-), do also exist. The osmium(VIII) species is strongly oxidising, but not nearly
as oxidising as its ruthenium analogue.
Coordination Numbers[6]
1.4.2
In terms of its coordination numbers, platinum group metals show very little versatility,
and osmium is no exception. The majority of the osmium complexes exhibit octahedral
geometries.
Table 1.1 illustrates the more unusual coordination numbers and
geometries. The table contains some examples of eight- and seven-coordination, in
addition to certain complexes that exhibit geometries ranging from square-based
pyramidal (primarily displayed by higher oxidation state osmium ions) to bipyramidal
(displayed by lower oxidation state osmium ions) geometries. Although the tetrahedral
geometry is displayed by osmium tetroxide, it is still considered as a rare geometrical
structure for osmium.
Table 1.1: The more unusual coordination numbers for osmium
Coordination
number
8
[6]
Examples
Geometry
Os(PMe2Ph)2H6
Unknown
Os(edta)(H2O)
Monocapped Octahedron
OsF7
Pentagonal bipyramidal
Os(Pet2Ph)4H3
Distorted Pentagonal bipyramidal
Os2O4(O2R)2
Square-based pyramidal
Os(CO)5
Trigonal bipyramidal
7
5
-
4
OsO4, [OsO4]
Tetrahedral
Os(NO)2(PPh3)2
Distorted Tetrahedral
8
1.4.3
Coordinating Ligands[6]
Group VII donor ligands
All of the halide ions (F-, Cl-, Br- and I-) form octahedral complexes with osmium ions.
Fluorides are associated with the VIII, VII, VI and V oxidation states while Cl-, Br- and Iare associated with the IV and III oxidation states.
Group VI donor ligands
The coordination chemistry of high oxidation state osmium (the VIII, VII and VI states)
are dominated by the oxo-species, of which the tetrahedral osmium tetroxide, OsO4, is
the most important. There are also a number of sulphur-donor complexes which forms
with these metal ions.
Group V donor ligands
Several nitrido complexes of osmium(VI) and osmium(IV) as well as the “osmiamate”
ion ([OsVIIIO3N]-) are now known to exist. Considerable work has been conducted on
the bipy, phen and terpy complexes of osmium. The ammine and ethylenediamine
ligands, which do not exhibit π-acceptor capabilities, form stable complexes with
osmium(IV) and osmium(III). On the other hand, phosphorous, arsenic and antimony,
with their good π-acceptor capabilities, form stable complexes with osmium(III) and
osmium(II)
Group IV donor ligands
This group is dominated by the osmium cluster carbonyl chemistry. As an exceptional σdonor, mild π-acceptor ligand, cyanide forms a rather stable complex with divalent
osmium, forming the Os(CN)64- species. In the trans-[OsO2(CN)4]2- complex, the high
oxidation state of osmium is stabilised by the oxo-ligands with its strong σ-, π-donor
capabilities.
9
1.5
Aims and Objectives
Osmium is of little use industrially (compared to other platinum group metals), which is
owed chiefly to its expense of refining and also the considerable difficultly of working
with the metal. Even though not as valuable, the mining industry still faces the task of
separating and stabilising osmium during the refining processes associated with the
procurement of the more valuable platinum group metals, including platinum and
palladium, amongst others.
In addition, this industry also has to deal with the
environmental and occupational health threats that osmium poses.
It is therefore
essential to acquire experience and knowledge in the chemical behaviour and handling
of osmium.
Osmium tetroxide catalysed oxidations of organic molecules are important in many
organic syntheses, and although these reactions have been investigated in the past,
there does not seem to be a consensus regarding the mechanism by which these
reactions proceed.
This study stems from a process used in the platinum refining industry during which
osmium tetroxide is reduced in an alkaline medium to osmium(VI) using industrial
ethanol as a mild reducing agent. Spectrophotometric techniques, in conjunction with
several computational methods, were used to investigate the kinetics and equilibria
surrounding the reduction of osmium tetroxide by several primary alcohols in a
hydroxide matrix. Through these investigations a mechanism explaining the acquired
data is proposed. This mechanism allows a greater understanding of the fundamental
chemical behaviour associated with these species.
10
CHAPTER 2:
Experimental
2.1
Apparatus
2.1.1
UV-Vis Spectrophotometric Recordings
UV-Vis spectra were recorded using a Perkin-Elmer Lambda 12, double beam UV-Vis
spectrophotometer, interfaced with the UV WinLab (Version 1.22) software package.
The spectra were recorded using the following settings:
o
cycle time (where applicable): 120 seconds
o
scan rate: 240 nm/min
o
slit width: 1 nm
In aid of consistency, paired quartz cuvettes, with a 1 cm path length, were used in all
spectral recordings made.
Kinetic investigations were conducted at 25°C. In order to maintain a constant room
temperature, a Samsung SH 122KG external air-conditioning unit was employed. This
unit showed ± 0.5°C deviation from the desired temperature.
In addition, a Grant KD100 circulating thermostat controller, mounted onto a Grant W6
water bath equipped with a cooling coil, was used to maintain the temperature of the
spectrophotometer cuvette holder at 25°C (± 0.1°C).
A pump, attached to the
thermostatic water bath, was used to circulate water through the rubber tubing, as
illustrated in Figure 2.1. The tubing extended through the cuvette-containing chamber
of the spectrophotometer, and is attached to a thermostatic beaker which contains the
sample solutions. This ensured that the contents of the beaker and the cuvettes were
at equal temperature. The contents of the beaker were magnetically agitated with a
Metrohm 128 stirrer.
11
Cuvette
Chamber
Beaker
Circulating
Pump
Magnetic
Stirrer
Thermostatic
Water bath
Computer
Aluminium-covered
Rubber Tubing
UV-Vis
Spectrophotometer
Figure 2.1: Illustration of the experimental system employed to record UV-Vis spectra at constant
temperatures
2.1.2
Mole Ratio Titrations
The absorbance measurements for mole ratio titrations were recorded with a
Metrohm 662 photometer. This photometer consists of a probe connected to the main
unit with two light guides. The first light guide relays light to the probe, which reflects
light back to the photometer unit through the second light guide. The end of the probe
is immersed into the reaction solution, therefore allowing titrations to be performed
without the removal of samples from the reaction solution. The light path through the
solution is 1 cm.
The photometer was connected via its analogue output to a titroprocessor.
This
enables photometric titrations to be performed automatically, making it possible to
record titrations with a large number of data points.
The main disadvantage of the photometer is that the absorbance can only be measured
at a single wavelength during a titration.
12
2.1.3
Potentiometric Titrations
Titrations were performed and recorded using a Metrohm 780 pH meter and Metrohm
665 Dosimat.
These titrations were performed automatically, with the measured
aliquots of titrant being delivered via the dosimat.
2.1.4
pH Measurements
pH was measured with a Metrohm 780 pH meter using a Metrohm 6.0232.100
combined pH glass electrode. The electrode was calibrated with pH 4.00 (Metrohm
6.2307.100) and pH 7.00 (Metrohm 6.2307.110) buffer solutions.
2.1.5
Potentiometric Measurements
Potential was measured with a Metrohm 780 pH meter using a Metrohm 6.0402.100
combined platinum-wire electrode.
2.1.6
Preparation of Solutions
The stock solutions used in this study were all prepared in a constant temperature room
set at 25°C (± 0.2°C). The room temperature was maintained by means of a Bürisch
thermostatic circulator linked to a Carel temperature control unit.
Type 1 quality water, achieved by employing a Millipore Simplicity water purification
system, was used for the preparation of aqueous solutions.
The system provides
polishing of water, removing any remaining contaminants from distilled water[7]. The
water used throughout this study was produced in this manner, and will hereafter be
referred to as distilled water.
13
2.2
Computer Hardware and Software
Table 2.1: Computer platform used for kinetic and equilibrium calculations
Processor
Motherboard
1.86 GHz Intel
Asus Deluxe P5B
Pentium Core 2
SLI, 1066 MHz
Duo, at 2.2 GHz
Front Side Bus
Processing
Platform
64 Bit
Memory Module
2 GB DDR2
The Windows XP compatible software used during this study include:
o
Word 2003
o
Excel 2003
o
SigmaPlot 9.0.1
o
ChemDraw Version 8
o
Visual Basic.Net
o
SimpleGraph (Author: Dr E C Hosten)
o
SPC-V-MR (Author: Dr E C Hosten)
o
KinEqui (Author: Dr W J Gerber)
o
GP 2 (Author: Mr T E Geswindt)
The programs written during this study are listed in Appendix 1 and are discussed in the
relevant chapters where they are applied. These programs were employed for the
simulation of experimental kinetic data.
14
2.3
Reagents Utilised
Osmium, in the form of the potassium osmate salt (K2[OsO2(OH)4]), was obtained in a
crude form from the Anglo Platinum Research Centre. The crude potassium osmate
salt was oxidised to the volatile osmium tetroxide during the preparation of a pure
osmium tetroxide solution.
Due to the solubility of osmium tetroxide in carbon
tetrachloride, carbon tetrachloride was used to trap the volatile tetroxide. Pure
potassium osmate salt was prepared through the recrystallisation procedure described
in Chapter 2.6.
Table 2.2: Reagents utilised during this study
Salts
Reagent
Chemical Formula
Percentage
Purity
Supplier
Thiourea
CH4NS2
98
Associated
Chemical
Enterprises (Pty)
Ltd
Potassium hydroxide
KOH
88
Minema Laboratory
Supplies (Pty) Ltd
Sodium hydroxide
NaOH
98
Merck Chemicals
(Pty) Ltd
Sodium tetraborate
Na2B4O7·10H2O
99
May & Baker
Liquids
Reagent
Chemical Formula
Butan-1-ol
CH3CH2CH2CH2OH
Percentage
Composition
99
Supplier
Merck Chemicals
(Pty) Ltd
15
Carbon tetrachloride
CCl4
99.5
Ethanol
CH3CH2OH
99.9
Hydrochloric acid
HCl
32
Methanol
CH3OH
99.9
Merck Chemicals
(Pty) Ltd
Minema Laboratory
Supplies (Pty) Ltd
SMM Instruments
(Pty) Ltd
Merck Chemicals
(Pty) Ltd
Associated
Chemical
Enterprises (Pty)
Ltd
Associated
Chemical
Enterprises (Pty)
Ltd
Nitric Acid
HNO3
55
Orthophosphoric acid
H3PO4
85
Perchloric acid
HClO4
70
Merck Chemicals
(Pty) Ltd
Propan-1-ol
CH3CH2CH2OH
99
Merck Chemicals
(Pty) Ltd
99.99
Spectrascan
Elemental
Standard,
TeknoLab A/S
Ammonium
hexachloroosmium(IV)
(NH4)2[OsCl6]
16
2.4
Standardisation Methods
2.4.1
Standardisation of Acids
Acid solutions were standardised against freshly prepared borax1 (sodium tetraborate)
solutions. The exact concentrations of the prepared acid and base solutions were in the
order of 1 × 10-3 mol/L. In order to retain the maximum number of significant figures,
the total volume of the titrant at the endpoint was 25 mL. Titrations were repeated until
concordant results were obtained.
2.4.2
Preparation of a Standard Sodium Hydroxide Solution
Sodium hydroxide pellets were dissolved in distilled water and the freshly prepared
solutions were titrated against standardised hydrochloric acid solutions. In order to
retain the maximum number of significant figures, the total volume of the titrant at the
endpoint was at least 25 mL. Titrations were repeated until concordant results were
obtained.
2.5
Preparation and Storage of Osmium Tetroxide
2.5.1
Introduction
Pure osmium tetroxide solutions were prepared through the oxidation of potassium
osmate.
The volatile osmium tetroxide was then trapped in carbon tetrachloride.
Carbon tetrachloride is the ideal solvent for the storage of osmium tetroxide due to the
fact that:
o
osmium tetroxide is significantly more soluble in carbon tetrachloride than in
water
o
the UV-Vis spectrum of osmium tetroxide in carbon tetrachloride does not
change as a function of time, indicative of the stability of osmium tetroxide in
carbon tetrachloride.
During preparatory procedures, it was found that only a limited number of oxidising
agents resulted in the production of a pure osmium tetroxide solution.
In most
instances, the reduced product of the oxidising agent, and occasionally the oxidising
1
Borax was used as a primary standard
17
agent itself, contaminated the solution. Oxidising agents that proved to be inappropriate
included sodium chlorate and chlorine, both of which produced contaminating chlorine
species in the scrub solution. The presence of the oxidising agent as a contaminant in
the scrub solution leads to an increase in the oxidising capabilities of the tetroxide
solution.
Due to the aforementioned contamination factors, hydrogen peroxide was
selected as the oxidising agent.
The hydrogen peroxide was acidified with
orthophosphoric acid in order to enhance its oxidising capacity.
2.5.2
Preparation Procedure
An illustration of the experimental system employed for the preparation of a pure
osmium tetroxide solution is shown in Figure 2.2.
Approximately 240 mL carbon
tetrachloride was transferred to Dreschel flask 2 and approximately 6 g of crude
potassium osmate was transferred to Dreschel flask 1. A hydrogen peroxide solution,
consisting of the following components:
o
45 mL distilled water
o
45 ml 85% orthophosphoric acid
o
10 ml 30% hydrogen peroxide
was then carefully transferred to flask 1.
Immediately following the addition of the
hydrogen peroxide solution, the glass tubes were connected to the Dreschel flasks.
18
Figure 2.2: Illustration of the experimental setup used during the preparation of a pure OsO4
solution
Hydrogen peroxide oxidised the potassium osmate to the volatile osmium tetroxide
(Dreschel flask 1). With the aid of a stream of air purging the contents of flask 1, the
tetroxide formed in this flask was forced through the glass attachments into Dreschel
flask 2, which contained the carbon tetrachloride trap solution. This procedure was
allowed to proceed over a period of 8 - 10 hours. Once the required time had elapsed,
the osmium tetroxide solution in flask 2 was transferred to a stoppered dark glass
container.
19
2.5.3
Preparation of Aqueous Osmium Tetroxide
Aqueous osmium tetroxide solutions were prepared by extracting osmium tetroxide from
a carbon tetrachloride stock solution into distilled water. The extraction process was
allowed to proceed for at least 1 hour prior to separation of the organic and aqueous
phases. Constant agitation of the mixture was provided by an automated orbital shaker.
Once the extraction period had elapsed and the two phases were allowed to separate,
the organic phase was removed. The aqueous phase was filtered through Whatman 41
filter paper (which was wetted with distilled water) in order to remove residual carbon
tetrachloride present in the aqueous phase.
2.6
Preparation of Potassium Osmate
Potassium osmate was originally obtained as a crude salt, used as a source of osmium
during the preparation of pure osmium tetroxide solutions. However, during specific
investigations it was imperative that the potassium osmate salt be of high purity. This
was achieved by dissolving the crude potassium osmate in a warm, 2 mol/L potassium
hydroxide solution.
This solution was then filtered under vacuum, allowing for the
removal of impurities.
The filtrate was allowed to cool in an ice bath and pure
potassium osmate was recrystallised through the addition of potassium hydroxide
pellets.
In certain instances ethanol was added to the filtrate containing excess
potassium hydroxide, to facilitate crystal formation by lowering the dielectric constant of
the filtrate solution.
Pure potassium osmate was also prepared by the reduction of aqueous osmium
tetroxide in the presence of excess potassium hydroxide in ethanol.
Once in crystalline form, the potassium osmate was filtered and dried under vacuum for
3 - 5 days.
20
2.7
Determination of Osmium Concentration – The
Thiourea Colourimetric Method
2.7.1
Introduction
Since the discovery of osmium in 1804, nearly a century and a half would have passed
before acceptable methods for its analysis were developed. During this period various
methods were proposed, including[9]:
o
osmium separation as a sulphide species, followed by ignition in hydrogen
o
reduction of osmium(VIII) with alcohol
o
separation as hydrous oxide, followed by reduction in hydrogen
o
the precipitation of osmium as an ammonium or potassium chloro-osmate
species
o
iodometric determination through the reduction of osmium(VIII) with iodide
o
the precipitation of osmium with strychnine sulphate
o
potentiometric titrations
These methods proved laborious and the results obtained through these methods
displayed significant discrepancies.
A viable method was presented in 1918 when
Chuguaev[11] found that an aqueous solution of osmium tetroxide, upon treatment with
thiourea and hydrochloric acid, produced a brilliant, rose-red coloured solution.
Continued investigations resulted in the isolation of red crystals from the reaction
mixture, which had a percentage composition corresponding to either the trivalent
[Os(NH2CSNH2)6]Cl3·H2O species or the tetravalent [Os(NH2CSNH2)6]OHCl3 species.
During his earlier work, Chuguaev proposed that the composition of the red solid was
composed of the [Os(NH2CSNH2)6]OHCl3 species. The tetravalent species was widely
accepted until 1953, when Sauerbrunn and Sandell rejected the claims made by
Chuguaev by conclusively proving that the composition of the red solid was in fact the
trivalent hexathioureaosmium(III) cation[8].
Even with the advancement of technology in the 21st century, assaying of osmium still
proves to be problematic. Inductively coupled mass spectrometry (ICP-MS) seems, at
first glance, to be an attractive method for the assay of osmium. This method does,
however, present problems including:
21
o
the replacement of all plastic components of the spectrometer which would
possibly exposed the osmium samples, for example plastic tubing, spray
chamber etc. This is due to osmium, in the form of osmium tetroxide, reacting
with the plastic components it comes into contact with. In order to prevent the
formation of osmium tetroxide, all osmium samples should be reduced to a
single, stable lower oxidation state without the loss of any osmium during the
process. The entire procedure itself proves to be rather cumbersome.
o
to find a single matrix which does not oxidise, reduce nor volatilise the osmium
samples
Electrothermal atomic absorption spectrometry (ETAAS) is another analytical technique
which could not be used for the assay of osmium, as it suffers from the same problems
as ICP-MS, namely the need for replacement of plastic components.
In addition,
ETAAS illustrates a lack of reproducibility of results, with some authors reporting the
relative standard deviation across three replicates as 19%[12]. These authors ascribed
the lack of reproducibility to the high volatility and the ease of decomposition of osmium
tetroxide and the Os – O bonds, which presumably decompose during the drying and
ashing stages.
Due to the aforementioned problems associated with ICP-MS and ETAAS, and the lack
of reproducibility of results these techniques suffer from, it was opted to investigate only
the thiourea colourimetric method for the assay of osmium samples.
22
2.7.2
The Effect of Varying Thiourea Concentration on the Formation of
the [Os(NH2CSNH2)6]3+ Species
2.7.2.1 Literature Review
Osmium tetroxide, upon treatment with excess thiourea in an acidic medium, reacts
according to the following relation[8]:
2OsO4 + 22NH2CSNH2 + 6H+ → 2[Os(NH2CSNH2)6]3+ + 5(NH2)(NH)CS2(NH)(NH2)
+ 8H2O
… 2.1
According to Relation 2.1, thiourea acts as both the reductant as well as the
coordinating ligand, with each osmium equivalent reacting with eleven equivalents of
thiourea and three equivalents of acid[8]. Sauerbrunn and Sandell[8] reported that the
reaction depicted by Relation 2.1 occurred rapidly (approximately three days at 25°C)
when osmium tetroxide was used. However, in strict contrast to osmium tetroxide, the
reaction between hexachloroosmium(IV), [OsCl6]2-, and thiourea under identical
conditions was found to be extremely slow (approximately eight days at 25°C).
2.7.2.2 Experimental Procedures
A 1.095 mol/L thiourea stock solution was prepared in an 8.484 mol/L HCl matrix.
Varying volumes of this stock solution was used to prepare thiourea solutions consisting
of the following concentrations in 25 mL:
o
0.0657, 0.1314, 0.2627, 0.3941, 0.4598, 0.5253, 0.5912, 0.6569, 0.7882 and
0.9196 mol/L
Concentrated HCl (32% m/v) was used to maintain the HCl concentration of these
solutions at 5.091 mol/L.
To each of these solutions, 0.500 mL ammonium
hexachloroosmium(IV) elemental standard was added to maintain the total osmium
concentration at 1.051 × 10-4 mol/L. These solutions were equilibrated at 25°C, and the
UV-Vis spectra of the solutions recorded daily until no significant changes in these
spectra were observed. The results are based on the final spectra recorded.
23
2.7.2.3 Results and Discussion
Figure 2.3 depicts the UV-Vis spectra of solutions containing a range of thiourea
concentrations, while the osmium and HCl concentrations were kept constant.
thiourea
concentrations
exceeding
0.394 mol/L,
the
At
characteristic
hexathioureaosmium(III), [Os(NH2CSNH2)6]3+, cations’ spectra are observed.
These
spectra illustrate a broad band at 550 nm and a sharp peak at 480 nm, with the absence
of the peaks at 370 and 325 nm. The spectra of the solutions containing lower thiourea
concentrations (less than 0.263 mol/L) illustrate two additional peaks at 370 nm and
325 nm.
1.0
0.0000 M
0.0657 M
0.1314 M
0.2627 M
0.3941 M
0.4598 M
0.5253 M
0.5912 M
0.6578 M
0.7889 M
Absorbance
0.8
0.6
0.4
0.2
0.0
300
350
400
450
500
550
600
Wavelength /nm
Figure 2.3: UV-Vis spectra illustrating the formation of the [Os(NH2CSNH2)6]
3+
species as a
function of thiourea concentration. The direction of the solid arrows indicates increasing thiourea
-4
concentration. [HCl] = 5.091 mol/L; [Osmium] = 1.051 × 10 mol/L; solutions were equilibrated for
8 days at 25°C
24
The peaks at 370 nm and 335 nm are ascribed to the presence of the
hexachloroosmium(IV) species. This conclusion is based on the spectrum of the pure
hexachloroosmium(IV) species, obtained from the solution prepared in the absence of
thiourea. Figure 2.3 illustrates that the spectrum of the pure hexachloroosmium(IV)
species display only two peak maxima, at 370 and 325 nm respectively. This correlates
with the presence of similar peaks in the spectra of solutions of low thiourea
concentration.
0.5
Absorbance at 490nm
0.4
0.3
0.2
0.1
0.0
0.0
0.2
0.4
0.6
0.8
1.0
-1
[Thiourea] /mol.L
Figure 2.4: The change in absorbance at 490 nm as a function of thiourea concentration,
indicating the large excess of thiourea required for the complete conversion of [OsCl6]
[Os(NH2CSNH2)6]
3+
2-
to the
species. The ratio of thiourea:osmium should be at least 4300:1 in order for full
2-
3+
conversion of [OsCl6] to [Os(NH2CSNH2)6] .
The [Os(NH2CSNH2)6]3+ cations’ spectra illustrated in Figure 2.3 stabilises only once the
thiourea concentration is truly in vast excess over osmium, and the spectra remain
relatively constant once a thiourea concentration of 0.460 mol/L or greater have been
reached. This observation is further illustrated in Figure 2.4, where the absorbance at
490 nm remains constant once the thiourea concentration reaches 0.460 mol/L. This
25
fact was used in the selection of the optimal thiourea concentration for preparation of all
subsequent analytical solutions.
2.7.3
The Effect of Varying Hydrochloric Acid Concentration on the
Formation of the [Os(NH2CSNH2)6]3+ Species
2.7.3.1 Literature Review
According to Sauerbrunn and Sandell[8] and as illustrated by Relation 2.1, in the
presence of excess thiourea, one equivalent of osmium tetroxide reacts with three
equivalents of acid:
2OsO4 + 22NH2CSNH2 + 6H+ → 2[Os(NH2CSNH2)6]3+ + 5(NH2)(NH)CS2(NH)(NH2)
+ 8H2O
…Relation 2.1
Relation 2.1 depicts the reaction as being dependent only on the thiourea and acid
concentrations. However, as observed by Cristiani et al[10] the kinetics of this reaction
also depends on the type of acid used.
These authors found that different acids
resulted in different observed rate constants being obtained, and concluded that the
kinetics of the reaction indicated a specific acid catalysis. In addition, Cristiani et al
found that, when using perchloric acid as the source of H+, Relation 2.1 proceeds
according to four main processes. All of these are acid dependent and only two of
these processes are thiourea dependent. The two thiourea dependent processes were
found to be faster than the two thiourea independent processes[10].
In contrast to the rapid reaction between osmium tetroxide and thiourea in HCl medium
(at 25°C), the reaction between hexachloroosmium(IV) and thiourea was found to be
exceedingly slow under identical experimental conditions. To date, no reports in the
literature have been obtained discussing the reduction of hexachloroosmium(IV) with
thiourea in the presence of perchloric acid. Reference has only been made to the
reduction of hexachloroosmium(IV) in HCl solutions[8].
26
2.7.3.2 Experimental Procedures
This investigation was performed in three parts:
a) The reduction of [OsCl6]2- by thiourea as a function of HCl concentration.
b) The reduction of [OsCl6]2- by thiourea as a function of HCl at constant ionic strength.
c) The reduction of osmium tetroxide as a function of HCl concentration at constant
ionic strength.
a) The following stock solutions were prepared in 250 mL volumetric flasks:
o
1.095 mol/L thiourea in distilled water
o
1.095 mol/L thiourea in a 6.753 mol/L HCl matrix
o
1.095 mol/L thiourea in an 8.484 mol/L HCl matrix
Solutions containing constant thiourea and osmium concentrations, with varying HCl
concentrations, were prepared in 25 mL volumetric flasks adding the following volumes
of reagents and filling with distilled water:
•
•
solutions varying in HCl concentrations from 0 to 3.000 mol/L
o
15 mL of the 1.095 mol/L thiourea stock solution prepared in distilled water
o
0.890 mL ammonium hexachloroosmium(IV)
o
varying volumes of 32% HCl to obtain the desired HCl concentrations
solutions varying in HCl concentrations from 4.052 to 4.500 mol/L
o
15 mL of the 1.095 mol/L thiourea stock solution prepared in a 6.753 mol/L HCl
matrix
•
o
0.890 mL ammonium hexachloroosmium(IV)
o
varying volumes of 32% HCl to obtain the desired HCl concentrations
solutions varying in HCl concentrations from 5.091 to 7.000 mol/L
o
15 mL of the 1.095 mol/L thiourea stock solution prepared in an 8.484 mol/L HCl
matrix
o
0.890 mL ammonium hexachloroosmium(IV)
o
varying volumes of 32% HCl to obtain the desired HCl concentrations
27
The UV-Vis spectra of these solutions were recorded daily until no significant changes
in the spectra were observed, which usually occurred after a period of approximately
eight days. The results are based on the final spectral recordings.
b) A 1.642 mol/L thiourea stock solution was prepared with distilled water in a 250 mL
volumetric flask. This solution was used to maintain the thiourea concentration at
0.657 mol/L, by transferral of 10 mL of the stock solution to 25 mL volumetric flasks.
Varying volumes of 32% HCl was added to these flasks in order to obtain the
required HCl concentrations. The ionic strength of these solutions was adjusted to
6 mol/L by addition of the required volume of 70% HClO4.
The osmium
concentration was fixed at 2.103 × 10-4 mol/L by addition of 1.000 mL of a
1000 mg/L ammonium hexachloroosmium(IV) elemental standard to each of the
volumetric flasks.
The flasks were filled to the mark with distilled water.
The
solutions were allowed to equilibrate over an eight day period at 25°C prior to the
recording of UV-Vis spectra.
c) Osmium tetroxide was obtained as a freshly prepared aqueous solution through the
extraction from a carbon tetrachloride stock solution into distilled water, as described
in Chapter 2.5.3.
A 1.642 mol/L thiourea stock solution was prepared in a 250 mL volumetric flask.
This solution was used to maintain the thiourea concentration at 0.657 mol/L by
transferral of 10 ml of the stock solution to 25 ml volumetric flasks. Varying volumes
of 32% HCl was added to these flasks to obtain the final HCl concentrations ranging
from 0.500 mol/L to 6.000 mol/L. Ionic strength adjustments were made through the
addition of the required volumes of 70% perchloric acid. The ionic strength of each
of these solutions was fixed at 6.000 mol/L.
The osmium concentration was
-5
maintained at 6.554 × 10 mol/L throughout the series by addition of the extracted
aqueous osmium tetroxide solution. These solutions were allowed to equilibrate for
three days at 25°C prior to the recording UV-Vis spectra.
28
2.7.3.3 Results and Discussion
The UV-Vis spectra depicting the reduction of hexachloroosmium(IV) by thiourea as a
function of HCl concentration is illustrated in Figure 2.5.
At increased HCl
concentrations, the characteristic [Os(NH2CSNH2)6]3+ species’ spectra are observed
This observation is based on the presence of the broad band at 550 nm and the narrow
peak at 480 nm as well as the absence of the peaks at 370 and 325 nm. The peaks at
370
and
325 nm
are
ascribed
to
the
incomplete
conversion
of
the
hexachloroosmium(IV) species to the [Os(NH2CSNH2)6]3+ species, which occurs at HCl
concentrations lower than 5.091 mol/L.
0.489 M
0.998 M
1.507 M
3.034 M
4.052 M
5.091 M
6.089 M
7.107 M
1.4
1.2
Absorbance
1.0
0.8
0.6
0.4
0.2
0.0
300
350
400
450
500
550
600
Wavelength /nm
Figure 2.5: UV-Vis spectra of the [OsCl6]
2-
reduction by thiourea as a function of HCl
-4
concentration. [Thiourea] = 0.657 mol/L; [Osmium] = 1.871 × 10 mol/L
The degree of conversion of hexachloroosmium(IV) to the [Os(NH2CSNH2)6]3+ species
as a function of hydrochloric acid concentration is also illustrated in Figure 2.6. At an
HCl concentration of 5.091 mol/L, the absorbance at 490 nm reaches a plateau. This
29
implies the total conversion of hexachloroosmium(IV) to the [Os(NH2CSNH2)6]3+ cation
at these HCl concentrations.
1.4
370 nm
490 nm
1.2
Absorbance
1.0
0.8
0.6
0.4
0.2
0.0
0
1
2
3
4
[HCl] /mol.L
5
6
7
8
-1
Figure 2.6: The change in absorbance at selected wavelengths as a function of HCl concentration.
-4
[Thiourea] = 0.657 mol/L; [Osmium] = 1.871 × 10 mol/L
The
reaction
between
hexachloroosmium(IV)
and
thiourea
to
form
the
[Os(NH2CSNH2)6]3+ cation could also be observed by the dramatic decrease in the
absorbance at 370 nm (a wavelength which have been established to represent the
presence of hexachloroosmium(IV) in solution) as the HCl concentration is increased. It
is interesting to note that the absorbance at 490 nm decreases at HCl concentrations
exceeding 5.500 mol/L.
This trend could be ascribed to the formation of the
[Os(NH2CSNH2)5Cl]2+ and [Os(NH2CSNH2)4Cl2]+ cations, based on the existence of the
iridium and rhodium analogues, which have been reportedly isolated as their respective
salts[8].
30
1.8
1.6
Pure [OsCl6]2-
1.4
Absorbance
1.2
1.0
0.8
5.250M HCl
0.6
0.4
0.750M HCl
0.2
0.0
300
350
400
450
500
550
600
Wavelength /nm
Figure 2.7: UV-Vis spectra depicting the reduction of [OsCl6]
2-
by thiourea as a function of HCl
concentration at an ionic strength of 6 mol/L. The solid arrows indicate the direction of increasing
-4
[HCl]. The [HCl] ranges from 0.750 mol/L to 5.250 mol/L; [Osmium] = 2.103 × 10 mol/L. The
2-
spectrum of pure [OsCl6] is included for comparison.
The UV-Vis spectra of the reduction of hexachloroosmium(IV) by thiourea as a function
of HCl concentration at constant ionic strength is illustrated by Figure 2.7. Since the
total H+ concentration of each of the solutions were adjusted to 6.000 mol/L, it was
expected that the hexachloroosmium(IV) species in all the solutions would be converted
to the [Os(NH2CSNH2)6]3+ species at the same rate, if the reaction occurs via a similar
mechanism to that depicted by Relation 2.1. However, the presence of peaks at 370
and 325 nm at HCl concentrations lower than 4.000 mol/L, indicates the presence of
hexachloroosmium(IV). Only at HCl concentrations exceeding 4.000 mol/L do these
peaks disappear.
31
0.9
370 nm
490 nm
0.8
Absorbance
0.7
0.6
0.5
0.4
0.3
0.0
0.2
0.4
0.6
0.8
1.0
Mole Fraction Cl-
Figure 2.8: The absorbance at selected wavelengths as a function of the mole fraction Cl
0.9
370 nm
490 nm
0.8
Absorbance
0.7
0.6
0.5
0.4
0.3
0
2
4
6
8
mole Clmole ClO 4
mole Cl-
-
mole ClO 4
Figure 2.9: The absorbance at selected wavelengths as a function of
32
Figure 2.8 illustrates the absorbance at the selected wavelengths as a function of the
mole fraction of Cl-. The absorbance at 490 nm shows a linear increase as a function of
the increasing mole fraction of Cl-, implying an increase in the formation of the
[Os(NH2CSNH2)6]3+ species.
Correspondingly, the increase in the Cl- mole fraction
results in a decrease in absorbance at 370 nm, correlating to the decrease in
hexachloroosmium(IV) as it is reduced to form the [Os(NH2CSNH2)6]3+ cation. This
conclusion is supported by Figure 2.9, which depicts the absorbance at the indicated
wavelengths as a function of the mole ratio,
mole ClAs the mole ratio increases
- .
mole ClO4
(effectively implying an increase in HCl), the absorbance at 490 nm increase
correspondingly as the [Os(NH2CSNH2)6]3+ species is formed. At the same time the
absorbance at 370 nm decreases as the hexachloroosmium(IV) species is reduced to
the [Os(NH2CSNH2)6]3+ species. At first glance it seemed as if the reaction between
hexachloroosmium(IV) and thiourea is dependent on the chloride ion concentration, but
further scrutiny reveals that it is the type of acid employed that drives this reaction, with
the rate of reduction of hexachloroosmium(IV) by thiourea increasing with increasing
HCl concentrations and decreasing HClO4 concentrations.
The reaction at constant ionic strength was repeated with osmium tetroxide as the
osmium source, the results of which are illustrated in Figures 2.10 and 2.11.
33
0.35
0.30
Absorbance
0.25
0.20
0.15
0.10
0.05
0.00
320
360
400
440
480
520
560
600
Wavelength /nm
Figure 2.10: UV-Vis spectra depicting the reduction of osmium tetroxide by thiourea as a function
of
HCl
concentration
at
an
ionic
strength
of
6 mol/L.
[Thiourea] = 0.657 mol/L;
-5
[Osmium] = 6.554 × 10 mol/L; [HCl] ranges from 0.500 mol/L to 6.000 mol/L
0.30
370 nm
490 nm
0.25
Absorbance
0.20
0.15
0.10
0.05
0.00
0
1
2
3
4
5
6
7
-1
[HCl] /mol.L
Figure 2.11: The change in absorbance at selected wavelengths as a function of HCl
concentration
34
The reaction between osmium tetroxide and thiourea do not exhibit any significant
differences between the UV-Vis spectra obtained over the range of HCl concentrations
investigated, as illustrated in Figure 2.10.
In addition, the absorbance at 370 and
490 nm remains relatively constant, irrespective of the HCl concentration (Figure 2.11).
This is due to the fact that the reduction of osmium tetroxide by thiourea is
predominantly dependent on the total H+ concentration, which was kept constant at
6.000 mol/L. This is also reflected by the rate at which equilibrium is established when
osmium tetroxide is reacted with thiourea, in comparison to the reaction between
hexachloroosmium(IV)- and thiourea; which was found to be three days for osmium
tetroxide, compared to the eight day period required by the hexachloroosmium(IV)
species.
These results are in contrast to those obtained from the reaction between
hexachloroosmium(IV) and thiourea.
The predominant reason for these differences
could the dissimilar fundamental properties of hexachloroosmium(IV) and osmium
tetroxide with the former exhibiting greater kinetic and thermodynamic stability than the
latter species.
Thiourea, in the presence of excess H+, would thus reduce these
species through different mechanisms, although the final product in both mechanisms
would be the trivalent hexathioureaosmium(III) cation. This would also account for the
longer equilibration time required for the reduction of hexachloroosmium(IV) by thiourea
as compared to the reduction of osmium tetroxide. The reduction and ligand exchange
for hexachloroosmium(IV) would be slower due to its aforementioned enhanced
stability.
Although there are uncertainties surrounding the hexachloroosmium(IV) reduction by
thiourea, when compared to analogous reactions involving osmium tetroxide (in addition
to the poor establishment of the type of bonding which occurs in the resultant products)
the thiourea colourimetric method remains an accurate, consistent and efficient method
for the assay of osmium. The use of hexachloroosmium(IV) as a standard can be
considered as a more accurate (and less hazardous) alternative to the classical thiourea
colourimetric method, in which osmium tetroxide was used.
The reason for the
increased accuracy of the method when using hexachloroosmium(IV) is due to the
35
decrease in the loss of osmium during sample preparation.
In contrast, osmium
tetroxide is partially lost as the tetroxide vapour during sample preparation.
The optimum thiourea and HCl concentrations were respectively found to be 0.657 and
5.091 mol/L, and duly selected for the determination of the total osmium concentration
for subsequent samples.
36
2.7.4
The Osmium-Thiourea Calibration Curve
2.7.4.1 Literature Review
The formation of the [Os(NH2CSNH2)6]3+ species as a function of both thiourea and HCl
concentration was established in Chapters 2.7.2 and 2.7.3. During these investigations
it was observed that the formation of the [Os(NH2CSNH2)6]3+ species requires a vast
excess of thiourea and HCl. Subsequently, the optimal thiourea and HCl concentrations
were found to be 0.657 and 5.091 mol/L, respectively. Furthermore, it was established
that at 25°C, an equilibration period of eight days is required for the complete
conversion of hexachloroosmium(IV) to [Os(NH2CSNH2)6]3+, as opposed to the three
day equilibration period required for the conversion of osmium tetroxide to
[Os(NH2CSNH2)6]3+.
According to Ayres and Wells[9], the osmium concentration range best exhibiting
linearity was 8 mg/L (4.210 × 10-5 mol/L) to 40 mg/L (5.257 × 10-4 mol/L). During this
study, the linear range was extended from 1 mg/L (5.257 × 10-5 mol/L) to 50 mg/L
(2.628 × 10-4 mol/L), by using an adaptation of the classic thiourea method.
2.7.4.2 Experimental Procedures
Standard solutions were prepared using an ammonium hexachloroosmium(IV) standard
[Spectrascan standard for spectroscopy by Teknolab A/S]. This elemental standard is
maintained in a 4.9% (1.559 mol/L) HCl matrix.
Several standard solutions were
-3
prepared using the 1000 mg/L (5.257 × 10 mol/L) osmium standard such that the final
thiourea and HCl concentrations were 0.657 and 5.091 mol/L, respectively. The total
osmium concentrations of the standard solutions were:
5.257 × 10-6, 1.051 × 10-5, 1.577 × 10-5, 2.628 × 10-5, 5.257 × 10-5,
7.885 × 10-5, 1.051 × 10-4, 1.577 × 10-4, 2.103 × 10-4 and 2.628 × 10-4 mol/L
These solutions were equilibrated over a period of eight days after which the UV-Vis
spectra of the solutions were recorded.
37
2.7.4.3 Results and Discussion
2.0
-6
5.257×10 M
-5
1.051×10 M
-5
1.577×10 M
-5
2.628×10 M
-5
1.5
5.257×10 M
-5
7.885×10 M
-4
Absorbance
1.052×10 M
-4
1.577×10 M
-4
2.103×10 M
-4
2.628×10 M
1.0
0.5
0.0
300
350
400
450
500
550
600
Wavelength /nm
Figure 2.12: UV-Vis spectra of the standard ammonium hexachloroosmium(IV)-thiourea solutions
recorded after 8 days at 25°C. [Thiourea] = 0.657 mol/L; [HCl] = 5.091 mol/L; the respective
osmium concentrations are noted in the figure.
The UV-Vis spectra of the standard solutions are depicted in Figure 2.12.
These
spectra illustrate peak maxima at 480 nm. Conventionally, the wavelength at which the
greatest change in absorbance occurs (in this case 480 nm) is employed during the
construction of a calibration curve. However, in this investigation the absorbance at
490 nm was used for the construction of an osmium calibration curve. This is due to the
fact that the calibration curve constructed from the data at 480 nm yields a larger
positive y-intercept in comparison to the curve constructed from absorbance data at
490 nm; the former being approximately eleven times greater than the latter.
38
1.2
y = 3750x + 9.0264 × 10 -5
R 2 = 0.9999
Standard Error = 0.0029
Absorbance at 490nm
1.0
0.8
0.6
0.4
0.2
0.0
0
50x10-6
100x10-6
150x10-6
200x10-6
250x10-6
300x10-6
[Osmium] /mol.L-1
Figure 2.13: The calibration curve obtained through the thiourea colourimetric method. Calibration
curve constructed from the absorbance data at 490 nm
The osmium concentration range demonstrating linearity was extended from
5.257 × 10-6 to 2.628 × 10-4 mol/L (Figure 2.13). At 490 nm, the [Os(NH2CSNH2)6]3+
species exhibits a molar extinction coefficient of 3750.6 L mol-1 cm-1.
39
CHAPTER 3
The Alcohol Reduction of Osmium(VIII) in
Hydroxide Medium
3.1
Introduction
There is fairly extensive literature on the use of osmium tetroxide in the oxidation of
organic molecules. In these examples, osmium tetroxide acts as a catalyst with the use
of a co-oxidant to regenerate the reduced osmium product back to osmium(VIII).
Notably, the literature centres on the economically important oxidation of alkenes to cisdiols. There has been renewed interest in these reactions since the 2001 Nobel Prize in
Chemistry was awarded to Barry K. Sharpless, amongst others, for his work on “chirally
catalyzed oxidation reactions”[17], which features the catalysis by osmium tetroxide of an
asymmetric dihydroxylation.
However, many of the reports in the literature on the
oxidation of alcohols are out-dated. This fact, together with the interesting nature of
some of the preliminary data obtained in this study prompted a fresh look at the
oxidation of aliphatic alcohols by osmium tetroxide.
Further studies were undertaken to understand the equilibria and kinetics of the high
oxidation state osmium species, rather than the role of the organic molecules in the
reaction. This study focuses on a process used in the platinum refining industry during
which osmium tetroxide is reduced in a basic medium to osmium(VI) using industrial
ethanol. It is therefore to be expected that a detailed understanding of the relevant
osmium species in solution is of interest to this industry.
This review provides a general background to the osmium tetroxide – alcohol reaction,
discussing previous studies in this field as well as related studies into the oxidation of
other organic molecules by osmium tetroxide.
40
Due to its industrial importance, the oxidation of alkenes to cis-diols by osmium
tetroxide is covered extensively in the literature.
The reactions between osmium
tetroxide and alcohols are, however, not well documented.
Singh et al made a
comprehensive
as
study of
the
use
of
osmium
tetroxide
a
catalyst
with
hexacyanoferrate(III) as a co-oxidant; using various substrates such as alcohols, diols,
carboxylic acids and ketones[1, 6, 18-24]. In spite of many published reports, there does
not seem to be consensus on the mechanisms of either the alkene or alcohol/carbonyl
reactions. As yet unresolved issues include the mechanism of the formation of an
osmium – substrate complex as well as the nature of this complex; although other
parameters such as the hydroxide ion concentration should also be considered. A
discussion on the alkene hydroxylation reaction will serve to open the discussion at
hand.
Criegee[25] performed crucial work for this important reaction, showing that alkenes react
with osmium tetroxide to yield the osmium(VI) complexes, OsO2(O2R) and, in some
instances, OsO(O2R)2. Subsequent IR and X-ray crystallographic studies formulated
these complexes as dimeric species[25]. The rate of these reactions was increased with
the addition of organic bases such as pyridine; an early example of ligand accelerated
catalysis[26]. Hydrolysis of any of these complexes produced the cis-diol, R(OH)2 [6].
A generally favoured mechanism for this reaction is attack by the oxygen attached to
the osmium(VIII) centre on the unsaturated double bond of the alkene leading to a sixelectron transition state[23,
27]
. The reaction then proceeds to a five-membered ring
which, upon hydrolisation, accounts for the exclusively cis-product.
C
C
O
+ OsO4
C
O
6π
Os
C
O
O
C
O
C
O
C
O
Os
Os
O
O
O
C
Figure 3.1: The generally accepted mechanism for the oxidation of alkenes to cis-diols
O
O
41
VeeraSomaiah et al[28] conducted kinetic studies on the oxidation of unsaturated organic
compounds by osmium tetroxide in a sulphuric acid/acetic acid medium.
These
investigators found evidence for a single reaction with a single rate constant and
proposed a mechanism similar to that depicted in Figure 3.1, in which the six-electron
complex is suggested as a transient transition state. There is no evidence for the
formation of a stable five-membered ring which is to be expected. This is in agreement
with the results reported by Singh[23], since the five-membered ring proposed in
Figure 3.1 would at best be transient due to unfavourable angular strain on the fivemembered
ring,
since
third
row
transition
metals
have
no
d2
tetrahedral
stereochemistry.
As tetra-substituted alkenes, or those having electron-withdrawing groups, generally
form monomeric diester complexes in non-reducing organic solvents, one could expect
to find a stable intermediate osmium(VI)-ester complex. Alkenes such as cyclohexene
form dimeric monoester complexes[23].
O
CH
O
O
O
O
Os
CH
O
O
CH
CH
CH
CH
O
Os
Os
O
O
(a)
O
O
CH
Os
O
(b)
O
CH
O
O
O
O
CH
O
CH
Os
CH
O
O
CH
(c)
Figure 3.2: (a) syn- and (b) anti- dimeric monoesters, (c) monomeric diester
In contrast to the belief that only the osmate ester would be produced in an organic
medium, Subbaraman et al[29] claimed to have followed the kinetics of formation of this
ester in aqueous medium. Two separate rate constants were reported for this reaction
– one for the formation of an osmate ester and one for the hydrolysis of the ester[29].
The results were obtained in three independent steps: (1) the kinetics of formation of the
ester was followed spectrophotometrically in basic medium; (2) the osmate ester was
42
prepared in organic medium, and (3) the kinetics of the ester’s hydrolysis was followed
in basic aqueous medium. In addition, it is worthy to mention the monodentate nature
of the organic moiety of the osmate esters, which were formulated as (RO)2OsO2L2
where L represents various monodentate ligands. These authors did not postulate on a
reaction mechanism.
The conventionally accepted mechanism depicted by Figure 3.1 was challenged by
Sharpless et al[30]. Low temperature experiments with chromyl chloride oxidation of
olefins yielded organic products that were not easily accounted for by the previously
accepted mechanism. A new reaction mechanism for this reaction was postulated by
Sharpless et al, which was to include other d0 oxo-transition metal species. It was
argued that for the conventionally accepted mechanism, the reaction must proceed via
direct attack by the organic reductant on the oxygen end of the oxo moiety (path b
below) implying the resonance form indicated in 2. However, the resonance structure is
better represented by 1 below, indicating the preferred reaction pathway, a.
Os
1
O
Os
a
O
R
Os
2
O
b
Figure 3.3: Proposed pathways (a and b) for the reaction of organic reductant (R) with osmium
tetroxide
This mechanism is analogous to the nucleophillic attack in carbonyl compounds that
occurs exclusively at the electropositive carbon atom, and not the oxygen atom. This
implies the formation of an organometallic osmium(VIII) intermediate. This intermediate
subsequently undergoes rearrangement during the rate-determining step (possibly to a
dimeric cyclic ester) which, on rapid hydrolysis, yields the product. As opposed to the
alternative mechanism, this scheme reduces angular strain on the intermediate ester
ring in the first step in the absence of pyridine.
Attack by pyridine results in the
reductive insertion of the Os – C bond of the metallacycle into an oxo group giving an
ester which, on reacting with more pyridine, produces OsO2(O2R)L2. This mechanism
has yet to be conclusively proven.
43
R
R
O
C
O
O
+ OsO4
C
N
Os
O
N
R
O
N
Os
R
O
O
O
O
[30]
Figure 3.4: The mechanism proposed by Sharpless et al
N
Os
N
O
R
O
O
N
Os
N
O
R
R
O
O
O
R
involving nucleophillic attack by the
C – C double bond on the electropostive osmium
It can therefore be seen that two mechanisms were proposed, each involving the
formation of intermediate species of undetermined composition.
There is similar uncertainty regarding the oxidation of alcohols by osmium tetroxide. A
study into the kinetics of the oxidation of various organic substrates including alcohols,
diols and α-hydroxy acids by osmium tetroxide in alkaline medium conducted by
VeeraSomaiah et al[28] concluded that the reaction was first order in osmium and
substrate. Whilst no evidence was found for the formation of a stable intermediate
complex, a mechanism involving an osmium(VIII) – alcohol transition state was
proposed. This mechanism was followed by hydride ion abstraction by the osmium(VIII)
in a slow step, as illustrated by the reaction scheme in Figure 3.5.
OsO4(OH)H2O + OH-
[OsO4(OH)2]2- + H2O
H
R
OH + OsO4*
C
H
H
R
... 1
H
R
C
H
OH
O
C
OH
O
O
O
... 2
Os
O
O
K
Slow
Os
R
C
O + OsO3 + H2O
... 3
O
O
H
*[OsO4(OH)2]
2-
H
written as OsO4 as per original authors
Figure 3.5: Proposed reaction scheme for the oxidation of alcohols by osmium tetroxide
[28]
44
These investigators found that an increase in hydroxide concentration caused an
increase in the rate of the reaction and that the order of the reaction in hydroxide
concentration was less than unity.
The products of the oxidised substrates were
identified as the corresponding aldehydes and ketones. The rate constants for the
various alcohols were determined and a selection is reproduced in Table 3.1.
Table 3.1: The second order rate constants for the oxidation of several alcohols by osmium(VIII) in
-4
-
a hydroxide matrix; [Osmium] = 3.16 × 10 mol/L, [OH ] = 0.05 mol/L, Temp = 305 K
Subtrate
3
-1
[28]
-1
k / ×10 L.mol .s
Methanol
4.85
Ethanol
51.2
Chloroethanol
15.3
n-propanol
54.8
2-propanol
69.8
n-butanol
102.3
Isobutabol
83.4
Benzyl alcohol
16100
These authors’ proposed hydride ion abstraction mechanism was substantiated by the
trends exhibited by the rates of the oxidation of various alcohols by osmium tetroxide,
where electron-donating substituents at the α-carbon should increase the rate of
oxidation, while electron-withdrawing substituents should retard it. This hypothesis was
backed by a Taft plot with ρ* = -1.91, the negative value of which the authors claim
supports their hydride ion transfer mechanism[28]. The reaction scheme proposed by
these authors is illustrated in Figure 3.5.
The same authors found that, although in acidic medium all organic substrates with
double bonds reacted, there was no reaction between osmium tetroxide and alcohols.
However, they did not propose any explanation for this phenomenon[28].
Even though none of these studies found evidence for a stable osmium – alcohol
complex, it should be noted that the olive-green osmium(VI) complex, K2[OsO2(Ome)4],
has been synthesised by the reaction of osmium tetroxide and potassium hydroxide in
methanol and characterised by infra-red and UV-VIS spectroscopy[6].
In addition,
45
Subbaraman et al found evidence for the formation of an osmium(VI)-thymine glycol
complex upon reaction of osmium(VI)-pyridine complexes with thymine glycols[31].
Glycols, however, would be expected to form more stable esters with osmium(VI) than
alcohols because of the cyclic, bidentate nature of the ester.
At this point it would be instructive to review the oxidation of alcohols by other oxidants.
It is of particular interest to determine whether the reaction is initiated by C – H or O – H
cleavage. Westheimer[32], for example, proved that the alcohol oxidation by chromic
acid required the initial participation of the O – H bond, i.e. chromate ester formation.
This was deduced from the observation that the rate of oxidation of 2-propanol is
approximately 1500 times faster than that of diisopropyl ether.
H
H2CrO4
R1
O
R1
O
C
C
O
Cr
OH
R2
H
H
R2
O
R1
O
O
C
+ H3O+ + Cr
R2
OH
O
O
H
H
Figure 3.6: Mechanism of alcohol oxidation by chromic acid (Westheimer’s “ester mechanism”)
[32]
In contrast to chromic acid, ruthenium tetroxide is assumed to react via abstraction of
the α-hydrogen without interaction with the O-H bond[33]. In addition, it reacts with
alcohols and ethers at approximately the same rate, thus providing further evidence for
the above mentioned mechanistic interpretation.
This argument was employed by Rankin et al[34] for the oxidation of alcohols by
permanganate. The proposed mechanism involved C – H bond cleavage on the basis
of a similarity in rates of various substituted benzyl alcohols and their equivalent benzyl
methyl ethers. The rationale behind these assumptions was that oxidation proceeding
via a C – H bond cleavage mechanism would exhibit similar rates for equivalent
alcohols and ethers, since the α-hydrogens in alcohols and ethers are electronically
identical - in each, the hydrogen atom is attached to a carbon atom that is attached to
an oxygen and other carbons or hydrogen atoms).
46
This concludes an extensive review of the literature on the oxidation of alcohols and
ketones by osmium tetroxide. In addition, this review served to include a broad variety
of closely related studies in order for comparative conclusions to be drawn.
3.2
Isosbestic Points
Isosbestic points arise when two or more absorbing species have equal molar extinction
coefficients at the same wavelength when the total concentration of the said species is
constant. The presence of an isosbestic point is usually ascribed to the presence of
only two absorbing species. This is due to the fact that the probability of more than two
species having equal molar extinction coefficients at the same wavelength is very small.
This is not to say that other species are completely absent, but these species might be
present in low concentrations such that it does not interfere with the absorbance
measured at that wavelength.
In other instances it is possible that multiple species could be present at appreciable
concentrations with molar extinction coefficients that are similar in magnitude to the
isosbestic wavelength, or the molar extinction coefficients are multiples of each other, or
that some species have comparatively low molar extinction coefficients. In these cases
isosbestic points would not be very useful, and the assumption that only two absorbing
species are present in appreciable concentrations during the formation of an isosbestic
points would be incorrect. These cases are normally difficult to evaluate; however,
careful experimentation and data analysis could reveal such anomalies.
Throughout this study, it is assumed that only two light absorbing species are present in
appreciable concentrations when reference is made to an isosbestic point.
47
3.3
The Stability of Osmium(VIII) in a 2M Hydroxide Matrix
3.3.1
Literature Review
Due to its central position within the third row of transition metals, osmium can attain
various oxidation states through the nature of the coordinating ligands, rendering its
chemistry both unique as well as dynamic. Due to the fact that the alcohol-osmium
tetroxide reactions are conducted in a hydroxide matrix, it only seems fitting to discuss
the stability of osmium tetroxide in basic medium.
Osmium tetroxide exists as a clear translucent solid, which sublimes at room
temperature (melting point = 40.25°C).
It is known for its high solubility in non-
coordinating media such as CCl4 (375 g per 100 g CCl4) and its moderate solubility in
water (7.24 g per 100 g water)[13]. Figure 3.7 illustrates the UV-Vis spectrum of osmium
tetroxide in both CCl4 and water. The complete spectrum of osmium tetroxide in CCl4 is
not shown due to the fact that CCl4 interferes with the osmium tetroxide spectrum at
lower wavelengths, creating background noise. This is ascribed to the symmetrical
structure shared by CCl4 and osmium tetroxide (i.e. both molecules are tetrahedral).
The spectrum of gaseous osmium tetroxide is included for comparison[14, 15].
48
3.0
Water
CCl4
2.5
Gaseous Phase
Absorbance
2.0
1.5
1.0
0.5
0.0
220
240
260
280
300
320
340
Wavelength /nm
Figure 3.7: The UV-Vis spectra of OsO4 in both CCl4 and water, obtained during this study. The
spectrum of gaseous OsO4 is included for comparison
[14, 15]
The spectra of osmium tetroxide in CCl4 and in water are similar to that of gaseous
osmium tetroxide.
The spectra of gaseous osmium tetroxide display structured
absorptions indicative of a highly symmetrical species with a low density of vibrational
states[35]. Absorption spectra of aqueous osmium tetroxide also illustrate vibrational
structure; however, the individual bands are broader than the spectra of gaseous
osmium tetroxide and osmium tetroxide in CCl4.
In alkaline media, osmium tetroxide expands its coordination number to form the
[OsO4(OH)]- and cis - [OsO4(OH)2]2- species[35]. Various studies have been reported to
establish the reaction equilibria of osmium tetroxide with hydroxide. These studies,
having examined the speciation of osmium tetroxide as a function of pH, are in general
concurrence, if not in quantitative agreement[16, 35].
49
According to these authors, the following reaction equilibria were established:
Ka1
OsO4·2H2O
[OsO4(OH)]-·H2O + H3O+
Ka2
[OsO4(OH)]-·H2O
…3.1
…3.2
[OsO4(OH)2]2- + H3O+
The reported acid dissociation constants for the osmium(VIII) acid, OsO4·2H2O, are
Ka1 = 8.69×10-13 and Ka2 = 7.58×10-15 M[35]. These values were used to construct a
species distribution diagram (Figure 3.2), from which predictions could be made to
establish which species would be present at a specified pH.
1.0
OsO4
-
[OsO4(OH)]
Mole Fraction Osmium
0.8
[OsO4(OH)2]
2-
0.6
0.4
0.2
0.0
8
10
12
14
16
pH
Figure 3.8: Species distribution diagram for OsO4 as a function of pH.
The distribution diagram depicted by Figure 3.8 was based on the rapid equilibrium
between osmium tetroxide and hydroxide, the latter acting as the coordinating ligand.
Figure 3.8 illustrates that an increase in pH leads to increased formation of the
[OsO4(OH)]- species. Significant amounts of the cis - [OsO4(OH)2]2- species only forms
at pH > 14, and under these conditions this species spontaneously decomposes to form
50
the trans-[OsO2(OH)4]2- species, which is considered as the dominant osmium(VI)
species in aqueous solutions above pH = 5 [35].
3.3.2
Experimental Procedures
An aqueous osmium tetroxide solution was prepared by extracting osmium tetroxide
from a CCl4 stock solution, as described in Chapter 2.5.3.
A 1.305 × 10-4 mol/L
osmium(VIII) solution was prepared through the addition of 20 mL of the extracted
osmium tetroxide to a reaction vessel containing 230 mL of a 2.174 mol/L NaOH
solution, such that the final hydroxide concentration was 2 mol/L (pH = 14.3).
The NaOH solution was prepared using degassed water and the solution was purged
with nitrogen prior to the addition of the aqueous osmium tetroxide solution. After the
addition of osmium tetroxide, the solution was maintained under inert conditions for the
duration of the experiment. This was done in order to exclude carbon dioxide from the
solution, thus preventing the formation of carbonates. Carbonate formation would lead
to a lowering in pH in addition to possible side-reactions involving the produced
carbonates.
The first UV-Vis spectrum was recorded immediately following the addition of osmium
tetroxide to the reaction vessel.
Subsequent spectra were recorded by periodic
sampling of the solution over a period of 625 hours.
A
100 mL stock osmium(VI) solution was
prepared by dissolving 0.3339 g
K2[OsO2(OH)4] in 2 mol/L NaOH solution such that the total osmium concentration was
9.062 × 10-3 mol/L. In order to attain a 1.305 × 10-4 mol/L osmium(VI) solution, 720 µL
of the stock osmium(VI) solution was transferred to a 50 mL volumetric flask which was
then filled with 2 mol/L NaOH solution.
The total osmium concentration of each individual solution was determined through the
thiourea method.
51
3.3.3
Results and Discussion
As illustrated in Figure 3.9 there is a substantial change, over time, in the optical
spectrum of osmium(VIII) in a 2 mol/L hydroxide matrix. The dashed arrow indicates
the initial osmium(VIII) spectrum at t = 0 hours, while the solid arrows indicates the
direction of increasing time.
0.5
Absorbance
0.4
0.3
0.2
0.1
t=0
0.0
300
400
500
600
Wavelength /nm
Figure 3.9: The change in the UV-Vis spectrum of osmium(VIII) in 2 mol/L NaOH as a function of
time, from t = 0 hour to t = 625 hours. The spectra change in the direction of the solid arrows over
-4
time. [Osmium] = 1.305 × 10 mol/L
In Chapter 3.3.1, it was concluded that the predominant osmium(VIII) species present at
pH = 14.3 is the cis - [OsO4(OH)2]2- species. This species has a characteristic UV-Vis
spectrum, which shows two peak maxima at 260 and 320 nm. As the reaction proceeds
as a function of time, these maxima show a general increase. This increase is also
associated with a shift in the peak maximum from 320 nm to 341 nm, and the eventual
disappearance of the peak at 260 nm. The absorbance in wavelength region 320 –
475 nm shows a general increase to the point where a plateau is reached, after which
52
the absorbance slowly decreases. In addition, the spectra also indicate two sets of
isosbestic points; the first being formed at 274 nm, which shifts to 258 nm as the
reaction progresses.
0.40
Absorbance 370nm
0.35
0.30
0.25
0.20
0.15
0
100
200
300
400
500
600
Time /Hours
Figure 3.10: Progress curve depicting the rate of change of absorbance at 370 nm.
-4
[Osmium] = 1.305×10 mol/L; [NaOH] = 2 mol/L
Figure 3.10 illustrates the change of absorbance at 370 nm as the reaction progresses.
This figure suggests that the absorbance increases, reaches a plateau, and then
decreases slowly until equilibrium is reached.
This general trend illustrates two
important points – (a) the reaction follows a distinct two-step process, and (b) there
must be at least three absorbing osmium species present in appreciable concentrations.
To elaborate on these points, various osmium species would have different molar
extinction coefficients at a single wavelength (excepting the isosbestic wavelengths).
The formation of an intermediate species, with a molar extinction coefficient larger than
the initial osmium(VIII) species would therefore result in an increase in the absorbance
at said wavelength; provided that the intermediate species is present in appreciable
53
concentration. This corresponds to the first step in the reaction process. The second
step in the reaction would correspond to the formation of a third species with a molar
extinction coefficient comparatively smaller than both that of the initial osmium(VIII) and
that of the intermediate species, thus leading to the observed decrease in absorbance.
However, it can also be argued that the observed changes in the optical spectrum is
only due to a combination of two species, namely the initial osmium(VIII) and the final
osmium product.
This uncertainty could be resolved experimentally.
Figure 3.11
illustrates UV-Vis spectra of the initial osmium(VIII) species and the experimentally
obtained “intermediate” species.
Since the reduction of osmium(VIII) leads to the
formation of osmium(VI) as the final product, the spectrum of a pure osmium(VI)
solution was recorded and is illustrated in Figure 3.11.
54
0.6
Os(VI)
Os(VIII)
Os-Intermediate
Addition Spectrum
0.5
Absorbance
0.4
0.3
0.2
0.1
0.0
300
400
500
600
Wavelength /nm
-4
Figure 3.11: The spectra isolated during the reduction of 1.305 × 10 mol/L osmium(VIII) in 2 mol/L
-4
NaOH. The spectrum of a 1.305 × 10 mol/L osmium(VI) solution in 2 mol/L NaOH is included for
comparison.
Since the Beer-Lambert law is additive, a theoretical addition spectrum between pure
osmium(VIII) and pure osmium(VI) can be constructed, as shown in Figure 3.11. If the
experimentally observed spectral changes were only due to the simultaneous presence
of osmium(VIII) and osmium(VI), then the theoretical addition spectrum would
correspond to that of the experimentally obtained “intermediate” species.
From
Figure 3.11 it is evident that this is not the case. It can thus be concluded that the
reduction reaction proceeds via a distinct intermediary species, which supports the
earlier hypothesis that the reduction of osmium(VIII) to osmium(VI) follows a distinct
two-step process involving at least three species.
55
3.4
The Reduction of Osmium Tetroxide by Aliphatic
Alcohols in a 2M Hydroxide Matrix
3.4.1
Literature Review
H.S Sing et al[18], who performed much of the early work on the kinetics of osmium
tetroxide catalysed oxidation of alcohols by hexacyanoferrate(III), found evidence for the
occurrence of only a single reaction with a single rate constant.
The flaw in many of
these early investigations was the fact that the first concentration recordings were made
only approximately 5 to 10 (and sometimes as much as 20) minutes after initiation of the
reaction, therefore excluding data during these first crucial minutes. They found that the
order of the reaction with respect to osmium, 1- and 2-propanol and hydroxide (at
hydroxide concentrations lower than 0.01 mol/L) was unity. At hydroxide concentrations
greater than 0.01 mol/L the rate became independent of hydroxide concentration.
These authors based their reaction scheme on the observation quoted from Cotton and
Wilkinson[36], that “OsO4(OH)22- is the only reactive species”.
However, at the low
hydroxide concentrations at which they were working there would be no OsO4(OH)22present. Even at the highest hydroxide concentration used (0.01 mol/L) there would be
approximately equal quantities of OsO4(OH)- and OsO4, but no OsO4(OH)22-.
attempt was made to explain why the
OsO4(OH)22-
No
is named as the only reactive
species. This assertion is repeated without explanation in other studies[21,
37]
. The
proposed reaction scheme involves a seven-coordinate osmium(VIII)-propanol transition
state (rare, in itself), which decomposes to the aldehyde and OsO2(OH)42-.
The
aldehyde is subsequently oxidised to the carboxylic acid.
These authors extended their work from propanol to cover many other alcohols, with
similar results and conclusions to the above study[19, 20, 23]. The derived rate laws were
similar for all these studies:
d[Fey] 2k 1K 1K 2[S][OH − ][Os(VIII) ]T
=
dt
1 + K 1[OH − ]
where: Fey is the hexacyanoferrate(III) co-oxidant;
S is the alcohol substrate
56
The rate and equilibrium constants refer to the following reaction scheme:
[OsO4(H2O)(OH)]- + OH[OsO4(OH)2]2- + S
Complex
k1
K2
K1
[OsO4(OH)2]2- + H2O
Complex
Os(VI) + Intermediate Products
Work with diethylene glycol monomethyl ether, diethylene glycol monoethyl ether,
methoxyethanol and ethoxyethanol[23] prompted them to conclude that the reaction
proceeds either by:
a)
activated complex formation between osmium tetroxide and the organic substrate
as discussed above; or
b)
activated complex formation between osmium tetroxide and an anion derived from
the alcohol molecule.
3.4.2
Experimental Procedures
This section serves to introduce the experimental procedure of a typical kinetic reaction
during which osmium tetroxide was reduced by several aliphatic alcohols at pH = 14.3.
Methanol was used as the representative alcohol.
The progress of the kinetic reactions was recorded using a Perkin-Elmer Lambda 12
UV-Vis spectrophotometer, interfaced with the UV WinLab software package.
Reactants were mixed in a thermostatic reaction vessel maintained at 25.0°C ± 0.1°C,
such that the final reagent volume was 25 mL. The reaction progress was followed in
one of two ways, depending on the reaction rate:
1) scanning the wavelength region from 600 nm to 200 nm at a cycle time of 2
minutes and a scan speed of 240 nm/min. This method was used to follow the
reaction at low methanol concentrations, during which the progress of the
reaction proceeded at a slow rate.
2) recording single wavelength absorbance data at 370 nm (the wavelength based
on the analysis of spectral curves that produced optimal progress curves).
Absorbance data was recorded at 0.5 second intervals over a period of 30
minutes. This method was used for reactions with a high methanol concentration
that resulted in faster reaction rates.
57
Prior to each kinetic reaction, a reaction “blank” was obtained, where the osmium(VIII)
spectrum in a 2 mol/L hydroxide was recorded in the absence of methanol. This was
required in order to obtain the initial osmium(VIII) spectrum at time equals zero minutes.
Osmium tetroxide was obtained as a freshly prepared aqueous stock solution, as
described in Chapter 2.5.3.
The osmium tetroxide was extracted from a carbon
tetrachloride stock solution into distilled water. Due to the volatile nature of osmium
tetroxide, coupled with its moderate solubility in water, the osmium tetroxide
concentration of the stock aqueous solution was expected to decrease over time. For
this reason, the total osmium concentration of each solution prepared for the kinetic
investigations were respectively determined by the thiourea method. In this manner the
recorded absorbance data for a series of alcohol concentrations could be normalised in
order to compensate for the gradual decrease in the osmium tetroxide concentration of
the stock aqueous solution.
All reactions were conducted in 2 mol/L hydroxide matrix.
This concentration was
achieved by the addition of an appropriate volume of a 6 mol/L sodium hydroxide
solution to the reaction vessel.
A 0.1716 mol/L stock methanol solution was prepared in a 2 mol/L sodium hydroxide
matrix. The solution was prepared in this manner in order to avoid a substantial change
in the pH of the osmium solution upon addition of the stock methanol solution.
Table 3.2 shows the reagent volumes and concentrations for a typical kinetic
investigation.
investigation.
All reagents were freshly prepared prior to commencement of the
58
Table 3.2: Reactant volumes and concentrations for the reaction of osmium tetroxide with
methanol in a 2 mol/L hydroxide matrix
Reaction Number
Reagent Volume
/Concentration
(1)
(2)
(3)
(4)
(5)
(6)
(7)
Volume 6M
NaOH / M
8.285
8.188
8.091
7.945
7.848
7.605
7.362
Volume
H2O / mL
14.570
14.375
14.181
13.890
13.695
13.210
12.724
0.146
0.437
0.728
1.166
1.457
2.185
2.914
2.000
2.000
2.000
2.000
2.000
2.000
2.000
1.000
3.000
5.000
8.000
10.00
15.00
20.00
2.558
2.558
2.558
2.558
2.558
2.558
2.558
Volume
0.1716 M
methanol in 2 M
NaOH / M
Volume
aqueous OsO4
/ mL
[Methanol]
-3
/ × 10 M
[Osmium]
-4
/ × 10 M
†
No more than 30 seconds elapsed from initiating the reaction to the first spectral
recording made.
This procedure was repeated for several aliphatic alcohols, including ethanol, propan-1ol, and butan-1-ol.
†
The calculated total osmium concentration after normalisation of UV-Vis data
59
3.4.3
Results and Discussion
Figure 3.12 illustrates the change in the osmium(VIII) UV-Vis spectrum as a function of
time; a trend typical of the osmium tetroxide-methanol reaction.
indicates the direction of increasing time.
The solid arrow
The spectra denoted by A, B and C
respectively refer to the spectra recorded at t = 0 minutes, t = 34 minutes and t = 986
minutes.
This figure shows the change in the shape of spectra, from the initial
osmium(VIII) spectrum to the spectrum obtained once equilibrium has been achieved.
0.6
B
A
0.5
Absorbance
0.4
0.3
0.2
C
0.1
0.0
240
280
320
360
400
440
480
520
560
600
Wavelength /nm
Figure 3.12: The change in the osmium(VIII) optical spectrum as a function of time, from t = 0 to
-3
t = 986 minutes, upon addition of 1.00 × 10 mol/L methanol. The spectra denoted by A, B, and C
respectively refer to the spectra recorded at t = 0, t = 34 and t = 986 minutes. The solid arrow
indicates the direction of absorbance change with time. The solid and dashed lines indicate the
-4
-
occurrence of two isosbestic points. [Osmium] = 1.305 × 10 mol/L; [OH ] = 2 mol/L
60
[a]
0 .5
A b s o rb a n c e
0 .4
0 .3
0 .2
0 .1
0 .0
230
270
310
350
390
430
470
510
550
590
510
550
590
W a v e le n g th /n m
[b]
0 .5
A b s o rb a n c e
0 .4
0 .3
0 .2
0 .1
0 .0
230
270
310
350
390
430
470
W a v e le n g th /n m
Figure 3.13: Illustration of the isosbestic points formed during the reduction of osmium tetroxide
by methanol in 2 mol/L hydroxide medium. (a) The first transient isosbestic point occurs at
274 nm as indicated by the dashed line; (b) the second isosbestic point occurs at 258 nm as
indicated by the solid line
61
The occurrence of two sets of isosbestic points is also evident in Figure 3.12. The first
transient isosbestic point occurs at 274 nm, which changes to the second isosbestic
point at 258 nm, respectively indicated by the solid and dashed lines in Figure 3.12.
Figures 3.13 (a) and (b) aims to provide a clearer illustration of these isosbestic points.
The occurrence of these isosbestic points hint at the establishment of at least two
equilibria involving a minimum of three high oxidation state osmium species.
Figure 3.14 shows the progress curves of the reactions reported in Table 3.2. The
absorbance data of the reaction with the lowest methanol concentration was recorded
by scanning the 600 nm to 200 nm wavelength region, at 2 minute cycle intervals over a
period of 986 minutes. The absorbance data of all the subsequent reactions reported in
Table 3.2 was recorded at a fixed wavelength (370 nm) at 0.5 second intervals.
It is clear that the absorbance at 370 nm initially increased as a function of time,
reached a plateau, and then gradually decreased until the reaction reached equilibrium;
a trend consistent with the reaction proceeding via a distinct two-step process. An
identical trend was established in Chapter 3.3.3 where the spontaneous reduction of
osmium(VIII) in a 2 mol/L hydroxide matrix was investigated. Once again it can be
proposed that the trend exhibited by Figure 3.8 implies that the reduction of
osmium(VIII) by methanol in a 2 mol/L hydroxide matrix proceeds via two consecutive
reactions which involves a minimum of three osmium species; a conclusion that was
drawn from the presence of isosbestic points.
62
0.65
-3
1.00×10 M
-3
3.00×10 M
-3
5.00×10 M
-3
8.00×10 M
-3
10.0×10 M
-3
15.0×10 M
-3
20.0×10 M
Absorbance at 370nm
0.60
0.55
0.50
0.45
0.40
0.35
0.30
0.25
0
500
1000
1500
2000
2500
Time /s
Figure 3.14: Progress curves demonstrating the rate of change of the absorbance at 370 nm at
-4
different methanol concentrations. [Osmium] = 2.631 × 10 mol/L; [NaOH] = 2 mol/L; Methanol
concentrations are denoted by the legend.
In addition, the similarities demonstrated by the spectral changes observed during this
investigation and that observed from the reduction of osmium(VIII) in a 2 mol/L
hydroxide matrix implies that the reduction of osmium(VIII) proceeds via identical
osmium species, irrespective whether the reducing agent is methanol or water.
Therefore, the same conclusions made in during Chapter 3.3.3 would be valid for this
reaction.
Figure 3.15 (a) illustrates three spectra isolated at various times during the reduction of
osmium(VIII) in the presence of methanol. The spectra depicted in Figure 3.15 (a) were
respectively recorded at a time where these spectra most closely represent:
1) the initial osmium(VIII) species (species A)
2) the product of the first reaction (species B)
63
3) the final product of the reaction
For clarity, these species were respectively labelled species A, species B and species
C.
[a]
[b]
0.6
0.6
Species A
0.5
t = 0 min
t = 34 min
t = 986 min
Species B
0.5
Species A
Species B
0.4
Absorbance
0.4
Absorbance
t = 0 hours
t = 262.3 hours
t = 624.1 hours
Pure Os(VI)
0.3
0.2
0.3
0.2
Species C
0.1
0.1
0.0
0.0
240
280
320
360
400
440
480
520
560
600
240
Wavelength /nm
280
320
360
400
440
480
520
560
600
Wavelength /nm
Figure 3.15:(a) Spectra isolated at various times during the reduction of osmium(VIII) by methanol.
-4
-3
[Osmium] = 2.631 × 10 mol/L; [Methanol] = 1.00×10 mol/L; [NaOH] = 2 mol/L. (b) A comparative
-4
reduction reaction conducted in the absence of methanol. [Osmium] = 1.305 × 10 mol/L;
[NaOH] = 2 mol/L. In both figures the times at which these spectra were recorded are denoted in
the legend.
Figure 3.15 is a comparison between those spectra obtained in Chapter 3.3.3 for the
reduction of osmium(VIII) in the absence of organic substrates.
The similarities
between these figures identified the observed spectra as osmium species as these
spectra could not be ascribed to the presence of any organic species nor its reaction
products.
This prompts the question whether the spontaneous reduction of osmium(VIII) in the
absence of methanol influenced the kinetics of the reaction in which methanol was
present.
Although this question will be addressed more rigorously in subsequent
chapters, preliminary assessments reveal that the reaction between osmium tetroxide
and hydroxide would not influence the validity of the kinetic reactions significantly. In
Figure 3.16, the reaction of osmium tetroxide with hydroxide is superimposed on the
progress curves obtained for the reduction of osmium tetroxide by methanol in a 2 mol/L
hydroxide matrix.
64
0.65
0.00 M
-3
1.00×10 M
-3
3.00×10 M
-3
5.00×10 M
-3
8.00×10 M
-3
10.0×10 M
-3
15.0×10 M
-3
20.0×10 M
Absorbance at 370nm
0.60
0.55
0.50
0.45
0.40
0.35
0.30
0.25
0
500
1000
1500
2000
2500
Time /s
Figure 3.16: Progress curves indicating the rate of change of the absorbance at 370 nm for
various methanol concentrations. The progress curve depicting the reaction of osmium(VIII) with
0 mol/L methanol was superimposed onto the progress curves for those reactions involving
-4
varying methanol concentrations. [Osmium] = 2.631 × 10 mol/L; [NaOH] = 2 mol/L; methanol
concentrations are denoted by the legend.
During the 30 minute period allowed for the osmium(VIII)-methanol experiments, there
is a noteworthy, albeit small, increase in the absorbance of the reaction conducted in
the absence of methanol. In terms of the kinetics of the reaction, the influence of the
spontaneous reduction osmium(VIII) in hydroxide matrix is negligible since the rate of
the methanol reduction would be orders of magnitude greater than that reaction
conducted in the absence of methanol. However, this is only a qualitative observation
and more accurate comparisons could only be made in subsequent chapters once the
rate constants of the various reactions had been established.
Figures 3.17 – 3.19 illustrate the progress curves obtained for the osmium-alcohol
reaction for the organic substrates used, i.e. ethanol, propan-1-ol and butan-1-ol.
65
0.65
-3
1.00×10 M
-3
3.00×10 M
-3
5.00×10 M
-3
8.00×10 M
-3
10.0×10 M
15.0×10-3 M
-3
20.0×10 M
Absorbance at 370nm
0.55
0.45
0.35
0.25
0.15
0
500
1000
1500
2000
2500
Time /s
Figure 3.17: Progress curves illustrating the change in absorbance at 370 nm as a function of time
for
the
reaction
between
osmium
tetroxide
and
varying
ethanol
concentrations.
-4
[Osmium] = 2.590 × 10 mol/L; [NaOH] = 2 mol/L; ethanol concentrations are denoted in the
legend.
0.55
-3
1.00×10 M
-3
5.00×10 M
-3
8.00×10 M
-3
10.0×10 M
-3
15.0×10 M
20.0×10-3 M
0.50
Absorbance at 370nm
0.45
0.40
0.35
0.30
0.25
0.20
0.15
0.10
0
500
1000
1500
2000
2500
Time /s
Figure 3.18: Progress curves illustrating the change in absorbance at 370 nm as a function of time
for
the
reaction
between
-4
osmium
tetroxide
and
varying
propan-1-ol
concentrations.
[Osmium] = 2.285 × 10 mol/L; [NaOH] = 2mol/L; propan-1-ol concentrations are denoted by the
legend.
66
0.55
-3
1.00×10 M
-3
3.00×10 M
-3
5.00×10 M
-3
8.00×10 M
-3
10.0×10 M
15.0×10-3 M
-3
20.0×10 M
0.50
Absorbance at 370nm
0.45
0.40
0.35
0.30
0.25
0.20
0.15
0.10
0
500
1000
1500
2000
2500
Time /s
Figure 3.19: Progress curves illustrating the change in absorbance at 370 nm as a function of time
for
the
reaction
between
osmium
tetroxide
and
varying
butan-1-ol
concentrations.
-4
[Osmium] = 2.212 × 10 mol/L; [NaOH] = 2 mol/L; butan-1-ol concentrations are denoted by the
legend.
Preliminary (qualitative) assessment of Figures 3.16 – 3.19 reveal that the oxidation of
alcohols by osmium tetroxide proceeded at a faster rate along the trend
methanol < ethanol < propan-1-ol < butan-1-ol. This trend can be explained in terms of
the increased electron donicity of the substituents on the α-carbon of the alcohols which
increases with increasing alcohol chain length (methanol < ethanol < propan-1ol < butan-1-ol), resulting in the observed reaction rate increase along this trend.
67
3.5
The Geometrical Analysis of Kinetic Data using
Mauser Diagrams
3.5.1
Literature Review
In virtually all aspects of physical chemistry the kinetics of chemical reactions is treated
only on the level of concentration equations.
Furthermore, most of these chemical
reactions in liquid phase are investigated via UV-Vis spectrophotometry, where the
measured output used is absorbance data, which generally obey the Beer-Lambert law.
In contrast to concentration determinations, absorbance data leads to a loss of kinetic
information due to its inherent limited sensitivity[38].
Mauser diagrams are a powerful tool used for the evaluation of absorbance data.
These diagrams are the collective name given to the absorbance (A), absorbance
difference (AD) and absorbance difference quotient (ADQ) diagrams.
These are
typically two-dimensional diagrams, with the so-called Mauser space being multidimensional (n ≥ 2)[39], which allow for the determination of the number (s) of linearly
independent reaction steps of a chemical reaction.
By definition, a linear reaction system consists of first-order reaction steps; while linearly
independent reactions are those reactions which are independent of the reaction order.
Each reaction mechanism consists of a distinct number (s) of linearly independent
reaction steps that can be determined through Mauser diagrams. Recently it has been
established that, in addition to obtaining the number of linearly independent reaction
steps, the geometric analysis of the “Mauser space” (or absorbance space) could
provide new routes for the kinetic evaluation of chemical reactions.
The absorbance (or absorbance differences) of n wavelengths establishes the axes of
the absorbance space.
A straight line in this space is obtained when the reaction
system consists of a single linearly independent reaction step (s = 1), with the reaction
order of the resultant curve being independent of the reaction order. A reaction system
being described by two linearly independent steps (s = 2) would lead to a bent curve in
68
the Mauser space, which lies on a single plane. Since the curve lies on a single plane,
a two-dimensional coordinate system can be introduced which lies in this plane. The
coordinates of the Mauser curve with regard to the established two-dimensional
coordinate system can thus be evaluated[39-43].
The reaction systems described by three linearly independent reaction steps (s = 3)
also leads to a bent curve in the Mauser space. However, these systems differ from
reaction systems with two linearly independent reaction steps in that the absorbance
curve obtained does not lie on a single plane, and thus a two-dimensional coordinate
system cannot be introduced. These reaction systems are evaluated on the basis of
three-dimensional absorbance diagrams (i.e. Ai versus Aj versus Ak; where the
subscripts refer to the respective wavelengths), using the concept of parallel
projection[42], during which three-dimensional absorbance diagram is geometrically
projected onto a two-dimensional coordinate system. In this manner, the eigenvalues
describing the reaction mechanism can be determined.
Furthermore, the reaction
system s = 3 is reduced to a system which is described by only two linearly independent
concentration variables[42].
The principles for the evaluation of the n-dimensional Mauser space is generally
applicable to reactions where s = 1, s = 2 and s = 3 for linear and non-linear reaction
systems.
The following relations illustrate examples of the reaction systems which
could be evaluated through the geometric analysis of the absorbance space[39, 42]:
for s = 1
for s = 2
A
B
A
A+B
B
A
A
A+B
A
; A
C
C
B
B ; C
C
D
C+D ;E
B ;B+C
F+D
D
69
for s = 3
A
A
A
A
A
B
D
C
B
C
D
D; E
B; C
B; C
D; E
B + C; D
E; F
F
F
G
E
A
B; C
D
F
Mauser diagrams provide particularly attractive routes for the elucidation of reaction
models and consequently reduce the number of unknown parameters associated with
the reaction. This is due to the fact that knowledge of the molar extinction coefficients
of the absorbing species is not a prerequisite for the application of the theory. The only
requirement for this type of evaluation is that a sufficient number of species absorb in
the region of interest, i.e. that the single reactions of the system are individually
registered spectrophotometrically. It should be noted that reaction models defined by a
specific number of linearly independent steps cannot be distinguished from one another
by purely spectroscopic means; e.g. for the system s = 3 the reaction model
A → B → C → D cannot be distinguished from the reaction model A → B; C → D;
E → F.
To elaborate on the evaluation of Mauser diagrams, consider the following general
consecutive reactions which are described by two linearly independent reaction steps:
A
k1
k-1
B
k2
k-2
C
… 3.3
From Relation 3.3 it could be assumed that, at any given time, only two absorbing
species are present in appreciable concentrations.
Figure 3.20 depicts a general Mauser diagram for a reaction system consisting of two
linearly independent steps, such as the reaction represented by Relation 3.3. The bent
curve illustrated in Figure 3.20, consists of two linear regions, denoted by regressions
[1] and [2]. Using the aforementioned assumption (i.e. that only two absorbing species
70
are present in appreciable concentrations at any given time) into consideration, two
important conclusions could be derived from Figure 3.20[44], namely:
o
the linear region denoted by the regression line denoted [1] represents the
reaction A ↔ B
o
the linear region denoted by the regression line denoted [2] represents the
reaction B ↔ C
2.2
C
2.0
B [2]
1.8
[3]
Absorbance j
1.6
1.4
1.2
1.0
B
0.8
0.6
A
[1]
0.4
0.2
0.0
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
Absorbance i
Figure 3.20: Typical 2-dimensional Mauser diagram for the general reaction A
↔B↔C
Extrapolation of the regression lines [1] and [2] results in a point where the two
regression lines intersect, which is denoted [3] in Figure 3.20. Effectively, this point
represents the absorbance of species B, at the respective wavelength i and j. At this
point the species in solution exists solely as species B, which implies that the
concentration of species B at this point would equal the concentration of species A at
the start of the reaction[44]. Since the concentration of species A at the start of the
reaction is usually known, the molar extinction coefficient of species B can be calculated
(at various wavelengths) using the Beer-Lambert law.
71
3.5.2
Experimental Procedures
An aqueous osmium tetroxide solution was prepared through extraction from a CCl4
stock solution into distilled water, as described in Chapter 2.
The total osmium
concentration of this solution was determined by the thiourea method.
Aqueous osmium tetroxide was transferred to a thermostatic reaction vessel containing
NaOH to give a final hydroxide concentration of 2 mol/L. This solution was prepared in
order to attain an initial osmium(VIII) spectrum (at time = 0 minutes), in the absence of
alcohol.
A second solution was prepared, in which aqueous osmium tetroxide was added to a
reaction vessel containing a mixture of NaOH and methanol, such that the final
hydroxide and methanol concentrations were 2 mol/L and 1.00×10-3 mol/L, respectively.
Once sufficient agitation had been achieved, the UV-Vis spectra of this solution were
recorded as a function of time. The time elapsed between the addition of osmium
tetroxide and the first spectral recording did not exceed 32 seconds.
The instrumental settings used for recording UV-Vis spectra were:
o
wavelength range scanned: 600 – 200 nm
o
cycle time: 2 minutes
o
scan speed: 240 nm/min
The reaction was stopped after 998 minutes.
3.5.3
Development of Computational Software for Data Analysis
The program GP2 was developed specifically for the geometric analysis of twodimensional Mauser diagrams.
This program allows for the absorbance of data
acquired at each wavelength to be plotted against the absorbance data of subsequent
wavelengths. For instance, the absorbance data at wavelength i is plotted against the
absorbance data acquired at every wavelength from wavelength (i + a) [where a = 1; 2;
3; …) to wavelength j; resulting in a total of [j - (i + 1)] Mauser diagrams being analysed
for wavelength i.
Employing a linear least-squares algorithm, the program fits two
regression lines to user-defined series for each of the Mauser diagrams generated at
72
wavelength i, in an analogous manner to the diagram depicted in Figure 3.20. The
coordinates for the point of intersect between the regression lines are then calculated
for each of the diagrams, and an average absorbance value is calculated at each
wavelength. The process is repeated for all subsequent wavelengths.
As previously mentioned, Mauser diagrams that produce a straight line in the Mauser
space describes a reaction system consisting of a single linearly independent reaction
step. In certain cases, reaction systems described by two linearly independent reaction
steps also produce Mauser diagrams in which a straight line is obtained in the Mauser
space. This phenomenon typically occurs when the wavelengths used to construct the
diagram are so close to each other (e.g. absorbance at i versus absorbance at (i + 1))
that the absorbance values of the respective wavelengths are indistinguishable from
each other. Consequently, the data obtained from these Mauser diagrams should be
considered as outliers, and should not contribute to the average absorbance returned
by the program. For this reason the program has an “angle filter” which excludes these
outlier values. In essence, the angle filter determines the angle formed at the point at
which the regression lines intersect. If the calculated angle does not fall within a userdefined range, the absorbance determined at that point is excluded and has no
contribution to the final average absorbance returned by the program for that particular
wavelength.
Consider Relation 3.3:
A
k1
k-1
B
k2
k-2
C
…3.3
The values returned by the GP2 program in this instance would represent the
absorbance of species B, since the species present at the point of intersect would be in
the form of species B. Since the concentration of species A at the start of the reaction
equals the concentration of species B at this point, the molar extinction coefficient of
species B at various wavelengths can be determined through the use of the BeerLambert law.
The source code of the GP2 program is detailed in Appendix 1.
73
3.5.4
Results and Discussion
Figure 3.21 illustrates the change in the UV-Vis spectra as a function of time for the
methanol reduction of osmium tetroxide in a hydroxide medium.
indicates the direction of increasing time.
The solid arrow
The spectra denoted by A, B and C
respectively refer to the spectra recorded at t = 0 minutes, t = 34 minutes and t = 986
minutes. This figure illustrates the general shape of the various spectra, from the initial
osmium(VIII) spectrum, to the final spectrum once the solution had reached equilibrium
(t = 986 minutes).
0.6
B
A
0.5
Absorbance
0.4
0.3
0.2
C
0.1
0.0
240
280
320
360
400
440
480
520
560
600
Wavelength /nm
Figure 3.21: The change in the Osmium (VIII) optical spectrum as a function of time, upon addition
-3
of 1.00 × 10 mol/L methanol. The spectra denoted by A, B and C respectively refer to the spectra
recorded at t = 0, t = 34 and t = 986 minutes. The solid arrow indicates the direction of increasing
-4
-
time. [Osmium] = 1.305 × 10 mol/L; [OH ] = 2 mol/L
74
Figure 3.22 [a] and [b] shows a three dimensional Mauser diagram which was
constructed from the data presented in Figure 3.21. The rotation of [a] results in the
formation of a straight line (as illustrated by [b]) which lies on a single plane, implying
that the number of linearly independent reaction steps equal two, i.e. s = 2.
[b]
[a]
0.38
0.38
0.36
0.36
0.34
0.28
0.26
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0.28
0.26
0.24
0.35
0.30
Absorba
0.25
0.20
nce 370
0.15
0.10
nm
0.20
0.45
0.40
0.35
0.30
0.25
0.20
0.15
0.10
ce
3
an
0.40
or
b
0.20
0.22
0.9
0.8
0.7
0.6
or
b
an
ce
24
0.22
70
n
0n
m
0.24
0.30
m
0.30
0.32
0.5
Absorbance
240nm
0.4
0.3
Ab
s
Absorbance 280nm
0.32
Ab
s
Absorbance 280nm
0.34
Figure 3.22: [a] 3D Mauser diagram of A370 vs. A240 vs. A280 (the indices indicate the wavelengths
used). [b] Rotation of part [a]. The curve lies on a single plane, and is viewed along the edge of
this plane. The result is a straight line indicating the case s = 2.
Since the bent curve observed in Figure 3.22 lies on a single plane, a two dimensional
coordinate system can be introduced which lies in this plane, as illustrated by
Figure 3.23.
Figure 3.23 illustrates a two-dimensional Mauser diagram for the reduction of
osmium(VIII) by methanol at pH = 14.3. This diagram hints at the occurrence of two
consecutive reduction reactions, as depicted in Figure 3.23. However, as will be seen
during the remainder of this chapter, the reduction reactions are not as simple as the
relations depicted in Figure 3.23.
75
1.0
i = 240nm
i = 280nm
Os(
VI )
Absorbance at i
0.8
Os
-In
0.6
0.4
Os(VIII)
t
Os-Int
0.2
0.0
0.15
0.25
0.35
0.45
0.55
Absorbance at 370nm
Figure 3.23: A 2D Mauser diagram constructed from the data presented in Figure 3.21.
The advantage of this type of analysis is that, irrespective of the nature of the
intermediate species, the molar extinction coefficient of the intermediate species could
easily be obtained, as described in Chapter 3.5.1. By employing the program GP2, the
molar extinction coefficients of the intermediate osmium species was calculated at
various wavelengths.
A molar extinction coefficient spectrum of the intermediate
species could thus be constructed, which is illustrated by Figure 3.24.
76
Molar extinction coefficient /L.mol-1.cm-1
7000
6000
5000
4000
3000
2000
1000
0
270
290
310
330
350
370
390
410
430
Wavelength /nm
Figure 3.24: Molar extinction spectrum for the Os(VII)-Intermediate species, calculated using the
program GP2.
It can be seen that this type of data analysis eliminates tedious experimental
procedures for the determination of the Os(VII)-intermediate species’ molar extinction
coefficient.
In addition, Mauser diagrams also aid in the elucidation of proposed
reaction models for the reduction of osmium(VIII) by methanol at pH = 14.3. The values
for the molar extinction coefficients obtained from this method would be compared to
those obtained using other methods of analysis in subsequent chapters.
77
3.6
The Osmium(VIII) – Alcohol Kinetic Model
3.6.1
Literature Review
The evaluation of kinetic data plays an important role in this study and it is thus
essential to briefly describe the theory behind some of the results.
The osmium(VIII) – alcohol reaction can be represented as:
x Os(VIII) + y RCH2OH → Products
…3.4
The rate of this reaction can therefore be written as:
Rate = -
d [RCH 2 OH]
d [Os(VIII)]
=−
= k [Os(VIII)] x [RCH 2 OH] y
dt
dt
…3.5
This rate equation can be reduced to a pseudo-first order rate equation by maintaining
the alcohol concentration in sufficient excess, thus incorporating the alcohol term into
the rate constant. Equation 3.5 can thus be reduced to:
Rate = -
d [Os(VIII)]
= k obs [Os(VIII)] x
dt
…3.6
If x = 1, the reaction is first order with respect to Os(VIII) and can thus be written as:
d [Os(VIII)]
= −k obs dt
[Os(VIII)]
…3.7
Equation 3.7 can now be integrated between time = 0 and time = t, using the Os(VIII)
concentration at time = 0 and time = t:
[ Os(VIII)] t
t
d [Os(VIII )]
= −k obs dt
[Os(VIII )]
0
[ Os(VIII)] 0
∫
∫
…3.8
Upon integration, equation 3.8 becomes:
ln [Os(VIII)] = kt – ln [Os(VIII)]0
…3.9
78
In an analogous manner, the linear rate equations for zero and second order reactions
(represented by equations 3.10 and 3.11, respectively) can be derived:
[Os(VIII)] t = [Os(VIII)] 0 − kt
…3.10
1
1
−
= kt
[Os(VIII)] t [Os(VIII)] 0
…3.11
The complexities of the osmium(VIII) – alcohol reaction prevents the plotting of kinetic
data
in
terms
of
equations 3.9 - 3.11.
straight
forward
single-step
reactions
demonstrated
by
Therefore, kinetic modelling software was utilised in order to
evaluate the kinetic data for the osmium(VIII) – alcohol reaction.
The fact that two
consecutive reduction reactions occur, imply that the equations cannot be plotted in
terms of two variables since, if the reactions occur simultaneously, there would be a
minimum of three absorbing species, each contributing to the total absorbance in
varying degrees at any given time. Thus, the utilisation of KinEqui kinetic modelling
software enabled fitting more complex reaction models to the experimentally obtained
kinetic data.
This section describes the challenges in proposing an appropriate reaction model that
fits the experimental data within acceptable statistical error. A number of kinetic models
were proposed; however, only four models that best fit the experimental data are
illustrated. A single theoretical model was chosen to represent the experimental data.
Reasons for this choice will be provided.
3.6.2
Experimental Procedures
Kinetic reactions were performed as previously discussed in Chapter 3.4.2, using
several aliphatic alcohols including methanol, ethanol, propan-1-ol and butan-1-ol.
79
3.6.3
Computational Software Utilised for Kinetic Modelling [45]
The program KinEqui (Visual Basic 6) was developed for the integration of rate
equations and the least squares fitting of the rate equations to experimental data. The
user interface of the program allows the user to tweak a number of parameters. The
program accesses a reaction model bank from which the user can select the
appropriate kinetic model. Reaction models not present in the model database could be
incorporated by introducing a new Visual Basic Script into the program without having to
recompile the entire program.
The calculation scheme for fitting spectrophotometric data is summarized below.
The program has two main components that work in tandem; namely a routine to
integrate the differential equations and a routine to do the least squares fitting, i.e.
function minimization. The least squares routine can be set to optimize only the rate
constants, only the extinction coefficients, or both simultaneously.
A Runge-Kutta algorithm[46] was used to integrate a set of rate equations (viz.
equation 3.12) numerically. The local truncation error at a specified step is a measure
of the amount by which the difference equation being used for the approximation fails to
satisfy the exact solution of the differential equation.
The Runge-Kutta method is
equivalent to a fourth order Taylor expansion method with the added advantage that the
functions do not need to be differentiated four times. The most dominant parameter
affecting the accuracy of the numerical solution is the time step (or space step) size, i.e.
the spacing of the mesh points where the true function is approximated. Instead of
defining local truncation error functions to test the accuracy of solutions, the technique
of decreasing step size is employed. The decrease in step size in each calculation
produces a more accurate approximation. This has the effect that the predicted solution
curve converges to a unique curve. This unique curve is an accurate representation of
the true solution curve within a negligibly small truncation error.
80
Let P be a set of first order differential equations.
P = { f1( t, x 1, x 2 ,........, x n ) , f2 ( t, x1, x 2 ,........, x n ) ,..........., fn ( t, x1, x 2 ,........, x n ) }
…3.12
dx i
= fi ( t, x 1, x 2 ,....., x i ,......., x n )
dt
…3.13
fi ( t ) = x i
initial condition for species i
…3.14
where: x = concentration
t = time
Integration of each function fi in Set P is represented in general by equation 3.15. The
differential equations in Set P are integrated simultaneously as described by Burden et
al[46].
y i (t ) =
t =n
∫ f ( t, x , x
i
1
2
,........, x n )dt
…3.15
t =1
In the program, the function yi(t) is not obtained analytically as equation 3.15 suggests,
but by using the Runge-Kutta difference equations.
The function yi(t) is, however,
useful to define here in order to facilitate further discussions relating to the least squares
calculation.
The predicted concentration yi(t) obtained from the numerical integration is compared
with the experimental absorbance or concentration data for minimization using a least
squares formulation as shown by equation 3.16.
S = ∑ ( A exp − ( A theo (1) + A theo ( 2 ) + A theo ( 3 ) + ......))2
…3.16
where S = error sum, yi(t) = ci
ATheo(i) = l∈ yi(t) for absorbance data
i
ATheo(i) = yi(t) for chromatography data set parameters, l = 1 and ∈ = 1
i
81
The Simplex algorithm was employed for the minimization of equation 3.16.
The
unknowns in the calculation are the molar extinction coefficients and the rate constants.
Each time the rate constants are updated in the least squares routine during a particular
iteration, it implies that a constant in the integration routine also changes. This has the
effect that the integration must be re-evaluated to calculate the new concentration
values of the species with different rate constants. Termination of the calculation is
controlled by the user who checks that a unique solution curve is obtained by
decreasing the step sizes and other tolerance parameters.
3.6.4
Results and Discussion
Models 1 – 4 reported below shows the theoretical fit of the models that were proposed.
A number of kinetic models were proposed; however, only four models that best fit the
experimental data is illustrated.
The best fit for each model returned a set of
corresponding molar extinction coefficients and rate constants. The molar extinction
coefficients and rate constants should, by definition, be constant across the entire range
of alcohol concentrations investigated. Due to possible experimental error, or because
of an inappropriate theoretical model being chosen, these parameters varied from one
alcohol concentration to another. Thus, the best-fit parameters were averaged across
the entire alcohol concentration range and the theoretical fit produced by these
parameters was superimposed onto the experimental data. The results reported below
only show that of methanol, although the data for all the alcohols that was investigated
were treated in an analogous manner. In each of the presented figures, the symbols
represent the experimental data and the lines represent the simulated kinetic fits.
82
Model 1
Os(VIII) + RCH2OH
Os(VII) + RCH2OH
k1
Os(VII) + RCHO
k2
Os(VI) + RCHO
5.00×10-3mol.L-1 Methanol
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
3.00×10-3mol.L-1 Methanol
0.65
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
Time /s
1500
2000
10.00×10-3mol.L-1 Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
8.00×10-3mol.L-1 Methanol
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
Time /s
1000
1500
2000
Time /s
15.00×10-3mol.L-1 Methanol
20.00×10-3mol.L-1 Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
1000
Time /s
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
Time /s
1000
1500
2000
Time /s
k1
k2
εOs(VIII)
εOs(VII)
εOs(VI)
0.665
8.470×10-2
1.140 × 103
2.694 × 103
1.185 × 103
83
Model 2
Os(VIII) + RCH2OH
Os(VII) + RCH2OH
-3
k+1
k-1
k2
Os(VII) + RCHO
Os(VI) + RCHO
-1
-3
0.60
0.60
0.55
0.55
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
0
2000
500
-3
-1
-3
8.00×10 mol.L Methanol
1500
2000
-1
10.00×10 mol.L Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
1000
Time /s
Time /s
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
0
2000
500
-3
1000
1500
2000
Time /s
Time /s
-1
-3
15.00×10 mol.L Methanol
-1
20.00×10 mol.L Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
-1
5.00×10 mol.L Methanol
0.65
Absorbance 370nm
Absorbance 370nm
3.00×10 mol.L Methanol
0.65
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
1000
1500
2000
Time /s
Time /s
k+1
k-1
k2
εOs(VIII)
εOs(VII)
εOs(VI)
0.202
30.348
7.134×10-2
1.193 × 103
4.971 × 103
0.753 × 103
84
Model 3
Os(VIII) + RCH2OH
Os(VI) + RCHO
k2
Os(VIII) + Os(VI)
Os2(VII) + RCH2OH
-3
k1
Os2(VII)
k3
2Os(VI) + RCHO
-3
-1
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
0.65
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
1000
-3
-1
-3
8.00×10 mol.L Methanol
2000
-1
10.00×10 mol.L Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
1500
Time /s
Time /s
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
1000
Time /s
-3
1500
2000
Time /s
-1
-3
15.00×10 mol.L Methanol
-1
20.00×10 mol.L Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
-1
5.00×10 mol.L Methanol
3.00×10 mol.L Methanol
0.65
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
Time /s
500
1000
1500
2000
Time /s
k1
k2
k3
εOs(VIII)
εOs2(VII)
εOs(VI)
0.423
440.079
4.815×10-2
1.197 × 103
4.879 × 103
0.926 × 103
85
Model 4
Os(VIII) + RCH2OH
Os(VI) + RCHO
k+2
k-2
Os(VIII) + Os(VI)
-3
k1
Os2(VII)
-1
-3
0.60
0.60
0.55
0.55
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
Time /s
-3
-1
1500
2000
-3
-1
10.00×10 mol.L Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
1000
Time /s
8.00×10 mol.L Methanol
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
500
Time /s
-3
1000
1500
2000
Time /s
-1
-3
15.00×10 mol.L Methanol
-1
20.00×10 mol.L Methanol
0.65
0.65
0.60
0.60
0.55
0.55
Absorbance 370nm
Absorbance 370nm
-1
5.00×10 mol.L Methanol
0.65
Absorbance 370nm
Absorbance 370nm
3.00×10 mol.L Methanol
0.65
0.50
0.45
0.40
0.50
0.45
0.40
0.35
0.35
0.30
0.30
0.25
0.25
0
500
1000
1500
2000
0
Time /s
500
1000
1500
2000
Time /s
k1
k+2
k-2
εOs(VIII)
εOs2(VII)
εOs(VI)
0.275
2154.47
8.697×10-2
1.151 × 103
6.397 × 103
0.908 × 103
86
The presented models represent the sum of the models fitted using KinEqui kinetic
modelling software.
These models produced the best theoretical fits, while other
models produced noticeably poor theoretical fits that were consequently discarded. At
this stage it is important to emphasize that there are certain components resulting in
experimental error that is inherent in these reactions. These errors include natural
experimental error inherent in measurement and agitation of each solution. In addition,
there is also an inherent error in the determination of the total osmium concentration.
However, it can be seen that these factors does not play a significant role, since the
average of the rate constants and molar extinction coefficients for Model 4 produced
exceptionally good theoretical fits across all methanol concentrations. Based on the
good theoretical fit obtained from this model, in addition to reasons which will become
apparent in this discussion, Models 1 – 3 were excluded and only Model 4 accepted.
Model 4 is mechanistically reasonable, in that one molecule of osmium(VIII) reacts with
one molecule of alcohol to exchange two electrons. The complexation reaction is in a
dynamic equilibrium, with a conditional equilibrium constant defined by:
K eq =
[Os 2 (VII)]
k
= +2
[Os(VIII)] × [Os(VI)] k −2
…3.27
This equilibrium constant will be quoted in Chapter 4 since it is an easily comparable
parameter, more so than forward and reverse reaction rate constants that may vary
within a set of experimental parameters.
This equilibrium reaction will favour the
products if the osmium(VIII) concentration is high. As the reaction proceeds, however,
the osmium(VIII) concentration becomes depleted and thus the equilibrium would tend
to favour the reactants. The magnitude of Keq is high, implying that the concentration of
the osmium(VIII) ascribed to this reaction will always be low.
The time elapsed from initiation of the reaction to the first spectral recording made (on
average 30 seconds) also produces a relative error margin, since it leads to a loss of
kinetic data during this time. This effect is negligible when methanol and ethanol are
used as reductants, but becomes more profound when propan-1-ol and butan-1-ol are
used since the rate of the reaction increases by orders of magnitude when using these
alcohols.
Therefore, the theoretical fits obtained for Model 4 are not as good for
propan-1-ol and butan-1-ol when compared to the fits obtained for methanol and
87
ethanol, which is illustrated by Figure 3.25 [a] and [b]. Table 3.3 summarises the kinetic
parameters obtained for all the alcohols investigated in this study.
[b]
[a]
0.5
0.65
0.60
0.4
Absorbance 370nm
Absorbance 370nm
0.55
0.50
0.45
0.40
0.3
0.2
0.35
0.1
0.30
0.25
0.0
0
500
1000
1500
2000
0
500
1000
Time /s
1500
2000
Time /s
Figure 3.25: Comparison between the theoretical fits obtained for [a] methanol and [b] propan-1ol, based on Model 4. The comparison illustrates the pronounced effect that a loss of kinetic data
has on the theoretical fit. Symbols = Experimental data; Lines = Theoretical fit.
[a] [Osmium] = 2.631 × 10
-4
mol.L ; [Methanol] = 15 × 10
-1
[b] [Osmium] = 2.285 × 10
-4
mol.L ; [Propan-1-ol] = 15 × 10
-1
-3
-1
mol.L .
-3
-1
mol.L .
Table 3.3: Calculated rate constants and molar extinction coefficients for the reduction of
osmium(VIII) by several primary alcohols at pH 14.3, based on Model 4
Extinction coefficient /
Rate Constants
Substrate
k1
k2
3
-1
× 10 L.mol .cm
k3
-1
ε Os(VIII)
ε Os2 (VII)
ε Os(VI)
8.732×10
1.151
6.397
0.908
34299.1
1.1417
1.109
6.392
0.932
1.206
93601.3
3.956
1.134
6.395
0.923
1.476
167279.6
7.971
1.134
6.392
0.929
-1
-1
-1
-1
-1
/ L.mol .s
/ L.mol .s
/s
Methanol
0.275
2154.5
Ethanol
1.070
Propan-1-ol
Butan-1-ol
-2
The molar extinction coefficient of the dimeric osmium(VII) intermediate species
calculated from Mauser diagrams are also in good agreement with that obtained using a
least squares fit, supporting the proposed reaction model. These results are compared
in Table 3.4.
88
Table 3.4: Comparison between the molar extinction coefficient (at various wavelengths) of the
Os2(VII) species calculated from a least squares [LS] method and Mauser diagrams [MD]
ε Os2 (VII) [LS] /
Wavelength / nm
3
-1
× 10 L.mol .cm
ε Os2 (VII) [MD] /
-1
3
-1
× 10 L.mol .cm
350
6.957
6.570
360
6.635
6.214
370
6.397
5.641
380
5.499
4.762
390
4.674
3.744
400
3.105
2.834
-1
The two dimensional Mauser diagrams can now be fully explained in according to the
proposed kinetic model. Figure 3.23 is reproduced here for more clarity.
1.0
i = 240nm
i = 280nm
Os(
V
Absorbance at i
0.8
Os
-Int
0.6
0.4
I)
Os(VIII)
Os-Int
0.2
0.0
0.15
0.25
0.35
0.45
0.55
Absorbance at 370nm
Figure 3.23: A 2D Mauser diagram constructed from the data presented in Figure 3.21.
In Chapter 3.5, it was reported that a bent curve lying on a single plane typically
represents the consecutive reaction A → B → C.
Figure 3.26 aims to provide an
89
interpretation of the two dimensional Mauser diagram which is more consistent with the
proposed kinetic model.
1.0
i = 240nm
i = 280nm
Absorbance at i
0.8
[2]
Os
(VI)
0.6
+ O
s2 (V
II)
Os 2(VII)
[1] Os(VIII) +
0.4
0.2
0.0
0.15
0.25
0.35
0.45
0.55
Absorbance at 370nm
Figure 3.26: 2D Mauser diagram interpreted in terms of the proposed kinetic model, Model 4.
The bent curve illustrated by Figure 3.26 can be interpreted in terms of the kinetic model
by considering that immediately following the formation of osmium(VI) in the first
reaction step, the produced osmium(VI) would react with osmium(VIII) to form a dimeric
osmium(VII) species.
The first linear region of in Figure 3.26 would therefore
correspond to the simultaneous presence of osmium(VIII) and the dimeric osmium(VII)
species. As the reaction proceed, the dimeric osmium(VII) complex is reduced to form
osmium(VI) and since osmium(VIII) becomes depleted, the predominant species would
now be the dimeric osmium(VII) complex and osmium(VI). The second linear region of
the Mauser diagram would thus correspond to the simultaneous presence of the
osmium(VII) dimer and osmium(VI). Although the alcohol reduction of osmium(VIII)
does not proceed via typical consecutive reduction reactions, the results obtained from
90
Mauser diagrams remain viable, and is corroborated by the good correlation with the
least squares analysis of kinetic data.
The next aspect of the reaction that must be decided is the reaction mechanism, i.e.
whether the reaction is initiated by C – H bond cleavage (as depicted in Figure 3.27) or
O - H bond cleavage (as depicted in Figure 3.28).
2-
OH
O
3-
OH
O
O
O
O
Os
OH
+ R
Os
O
OH
H
2-
O
H
O
OH
H2O
C
O
HO
OH
Os
OH
HO
O
H
C
R
O
H
OH-
Figure 3.27: The E2 C – H bond cleavage reaction mechanism
At first glance, there are certain aspects that impact negatively on the O – H bond
cleavage mechanism. Firstly, the RO- species would illustrate greater reactivity toward
the osmium(VIII) centre when compared to the ROH species. CH3O-, for example, is
approximately 20 000 times more reactive as a nucleophile when compared to CH3OH.
There is thus a definitive correlation between the rate of the reaction and the acidity of
the reacting nucleophile. The more acidic a nucleophile, in this case the alcohol, the
faster the rate of the reaction. In other words, since the acidity of the alcohols increases
in the order secondary alcohol < primary alcohol < methanol, the rate of the reaction
should increase in this order. In contrast to this prediction, the observed reaction rates
illustrate quite the opposite trend, with the methanol reaction being by far the slowest.
This reason led to the initial conclusion that the reaction might proceed via C – H bond
cleavage as illustrated in Figure 3.27.
The osmium(VIII) centre, being a d0 species and a strong electrophile, abstracts a
hydride ion from the alcohol in an E2 reaction, leading to an instant rearrangement of
the hydrogen atom to an oxygen atom in the second step of the proposed reaction
91
mechanism. This leads to further interaction with water in the final step to form the
osmium(VI) product.
High oxidation state osmium species are normally associated with strong σ - and π –
donor ligands, since these ligands would form stable complexes with ions possessing
few or no d-electrons. Oxygen is thus an excellent ligand for the d0 osmium(VIII) ion
and the initial association of the alcohol through the oxygen atom is far more favourable
than through a hydrogen atom.
2-
OH
O
O
O
Os
O
O
O
O
Os
HO
OH
H
H
+ R
Os
C
OH
O
H
H
C
H
OH
HO
HO
O
2-
O
H2O
O
H
O
2-
OH
C
H
OH-
R
R
Figure 3.28: The hydride transfer reaction mechanism – from the associative reaction of the
primary alcohol molecule with the osmium(VIII) centre, leading to the formation of the osmate ion
and the aldehyde.
The increase in the reaction rate, despite the decreasing acidity of the alcohol, can be
envisaged if the first step in the reaction mechanism depicted in Figure 3.28 is not the
rate limiting step of the reaction mechanism. If the second step of the depicted reaction
mechanism is considered as the rate limiting step, then the parameters governing its
rate will be manifested through empirical studies. The second part of the O – H bond
cleavage model depicts the association of the osmium(VIII) centre with the alcohol
molecule through an Os – O bond to form a large, low-charge molecule. Subsequently,
this molecule undergoes hydride ion transfer to the osmium centre, in which the hydride
attached to the α – carbon is transferred to the osmium with the resultant formation of a
C – O double bond and the reduction of osmium(VIII) to osmium(VI). There is a rapid
rearrangement of the hydrogen atom to an oxygen atom in the second step of the
O
92
depicted reaction mechanism, and then further interaction with water in the final step,
resulting in the formation of the osmium(VI) product.
The fact that the hydride ion transfer is considered to be the rate limiting step of this
reaction mechanism, infers that the more stable molecule in the absence of the hydride
ion would result in an increase in the reaction rate. Abstraction of the hydride ion
results in the establishment of a positive charge on the α – carbon of the alcohol
molecule. The larger and more polarisable alkyl groups attached to the α – carbon, the
more the electron density can shift toward that transient positive charge, resulting in a
lower energy transition state. This interpretation also elucidates the increase in the
reaction rate with the increasing number of alkyl groups attached to the α – carbon.
93
CHAPTER 4
The Osmium(VIII) – Osmium(VI)
Complexation Reaction
4.1
Introduction
The oxides of osmium in oxidation states VI or VIII act as a catalyst (in aqueous alkaline
medium) in
the oxidation of
alcohols by oxidants
such as
oxygen[60],
or
hexacyanoferrate(III)[18]. In these reactions, the transformation can be interpreted as a
cyclical process involving the reduction of osmium(VIII) by alcohol to osmium(VI),
followed by re-oxidation of the latter species to osmium(VIII).
Reports in literature
suggests the involvement of a monomeric osmium(VII) species[59].
In the previous chapter, least-squares fits to experimental kinetic data excluded the
possibility of the involvement of a monomeric osmium(VII) species. This was based on
the poor fits obtained from models which included the monomeric osmium(VII) species
as an intermediate species during the oxidation of primary alcohols by osmium(VIII). In
addition, the best fit model for the osmium(VIII) – alcohol reaction was given by:
Os(VIII) + RCH2OH
Os(VIII) + Os(VI)
k1
k+2
k-2
Os(VI) + RCHO
Os2(VII)
In none of the reviewed literature was reference made to the possibility of the formation
of a dimeric osmium(VII) intermediate species. It was therefore decided to investigate
the relationship between osmium(VIII) and osmium(VI) in greater detail, with the aim of
gaining further insight into the identity of the intermediate osmium(VII) species.
It
should be noted that the spectrophotometric techniques discussed here cannot
determine the oxidation state of the intermediate species.
94
4.2
The Stability of Osmium(VI) in a 2M Hydroxide Matrix
4.2.1
Literature Review
During the preparation of potassium osmate solutions in a 2 mol/L sodium hydroxide
matrix, it was observed that the colour of the osmate solution changed from purple to
brown as a function of time.
This prompted the investigation into the stability of
potassium osmate at pH 14.3 as a function of time.
In addition to the observed colour change, potassium osmate was also observed to
have a greater solubility in a hydroxide matrix than in water. The reported solubility of
potassium osmate in a potassium hydroxide matrix is 2 × 10-2 mol/L in 0.7 mol/L
potassium hydroxide at 276 K [47].
A change in the electronic spectrum of an osmate solution, as a function of hydroxide
concentration, was documented by Mouchel and Bremard[48]. This was interpreted as a
change in the species from the osmate ion, [OsO2(OH)4]2-, in less alkaline solutions to
the trioxo ion, [OsO3(OH)3]3-, in more alkaline solutions. These authors subsequently
calculated the electronic spectra of both these species and the value of the acid
constant at 298 K was calculated to be 9.64 × 10-16.
4.2.2
Experimental Procedures
K2[OsO2(OH)4] was purified and recrystallised from crude potassium osmate using the
method described in Chapter 2.6.
A 1.320 × 10-2 mol/L osmate solution was prepared by weighing and quantitatively
transferring 1.2233 g of purified K2[OsO2(OH)4] salt to a 250 mL volumetric flask. A
500 mL 2 mol/L sodium hydroxide solution was prepared with degassed water. The
sodium hydroxide solution was subsequently purged with nitrogen prior to its use. The
K2[OsO2(OH)4] salt was dissolved and diluted to 250 mL by addition of the freshly
prepared 2 mol/L sodium hydroxide stock solution.
95
A study of the stability of K2[OsO2(OH)4] in a 2 mol/L sodium hydroxide matrix was
conducted in two parts:
a) the stability of K2[OsO2(OH)4] in an oxygen atmosphere
b) the stability of K2[OsO2(OH)4] in a nitrogen atmosphere
a) Approximately 10.00 mL of the stock osmate solution was transferred to a reaction
vessel containing 240 mL of a 2 mol/L sodium hydroxide solution.
The sodium
hydroxide solution was prepared with degassed water, and the solution was purged
with oxygen for at least 15 minutes prior to the addition of the osmate stock solution.
The solution was continuously purged with oxygen over a six hour sampling period.
b) Approximately 10.00 mL of the stock osmate solution was transferred to a reaction
vessel containing 240 mL of a 2 mol/L sodium hydroxide solution.
The sodium
hydroxide solution was prepared with degassed water, and the solution was purged
with nitrogen for at least 15 minutes prior to the addition of the osmate stock
solution.
The solution was continuously purged with nitrogen over a six hour
sampling period.
UV-Vis spectra of the respective solutions were recorded immediately following the
addition of the osmate stock solution to the reaction vessels. Thereafter, spectra were
periodically recorded over a six hour period.
Following the initial six hour sampling period, these vessels were sealed, ensuring that
the solutions were kept saturated with oxygen and nitrogen, respectively. This was
done to minimise the loss of solution due to evaporation; while keeping the solutions
under oxidising and inert conditions, respectively. Subsequent spectra were recorded
by periodic sampling of the solution over a period of 48 hours.
The total osmium concentration of both solutions was determined by the thiourea
method as described in Chapter 2.
96
4.2.3
Results and Discussion
Figure 4.1 illustrates the change in the UV-Vis spectrum of osmium(VI) upon exposure
to an oxygen atmosphere as a function of time. Initially there is a significant increase in
the in absorbance, after which the absorbance increases very slowly.
The figure
demonstrates an overall change in the shape of the osmium(VI) spectrum, with the peak
maxima at both 300 and 350 nm increasing in intensity. However, the peak at 350 nm
undergoes a larger change with time.
Figure 4.2 illustrates the change in absorbance at 300 and 350 nm as a function of time.
This figure clearly show a significant initial increase in the absorbance at these
wavelengths, which is followed by a comparatively slow increase in absorbance as the
reaction reaches equilibrium.
2.0
t = 2868 min
Absorbance
1.5
1.0
0.5
t = 0 min
0.0
250
300
350
400
450
500
550
600
Wavelength /nm
Figure 4.1: The change in the UV-Vis spectrum of [OsO2(OH)4]
2-
upon exposure to an oxygen
atmosphere as a function of time. The dashed arrows respectively depict the spectra recorded at
t = 0 min and t = 2868 min. The solid arrow indicates the direction of increasing time.
-4
[Osmium] = 5.278 × 10 mol/L; [NaOH] = 2 mol/L
97
2.0
300 nm
350 nm
1.8
Absorbance
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0
500
1000
1500
2000
2500
3000
Time /min
Figure 4.2: The change in absorbance at 300 and 350 nm as a function of time under oxygen
-4
atmosphere. [Osmium] = 5.278 × 10 mol/L; [NaOH] = 2 mol/L
A comparative study was conducted under inert conditions (a nitrogen atmosphere), the
results of which is illustrated by Figures 4.3 and 4.4. From these figures it is evident
that the osmium(VI) species remain stable, under inert conditions, since no change in its
UV-Vis spectrum was observed over time.
The fact that no changes were observed in the UV-Vis spectrum of the osmium(VI)
species under inert conditions, implies that the exposure of osmium(VI) to oxygen
results in the oxidation of osmium(VI) to, presumably, the osmium(VIII) species,
[OsO4(OH)2]2-. The pH at which this investigation was conducted (pH 14.3) makes it
unlikely that the osmium(VI) species disproportionate; a finding corroborated by
Galbacs et al[47].
These authors asserts that disproportionation of osmium(VI) only
occurs at a pH below 10. In addition, the disproportionation of osmium(VI) is also
accompanied by the formation of a black precipitate[47] that was not observed in this
study.
98
2.0
Absorbance
1.5
1.0
0.5
0.0
250
300
350
400
450
500
550
600
wavelength /nm
Figure 4.3: The change in the UV-Vis spectrum of [OsO2(OH)4]
2-
upon exposure to a nitrogen
-4
atmosphere. [Osmium] = 4.578 × 10 mol/L; [NaOH] = 2 mol/L
300 nm
350 nm
1.6
Absorbance
1.4
1.2
1.0
0.8
0.6
0.4
0
500
1000
1500
2000
2500
3000
Time /min
Figure 4.4: The change in absorbance at 300 and 350 nm as a function of time under nitrogen
-4
atmosphere. [Osmium] = 4.578 × 10 mol/L; [NaOH] = 2 mol/L
99
The qualitative assessments made in this investigation provided invaluable information
surrounding the experimental procedures required to prepare a stable osmium(VI)
species in a 2 mol/L sodium hydroxide matrix.
All the osmium(VI) solutions were
subsequently prepared under inert conditions, ensuring that the osmate was not
oxidised by exposure to atmospheric oxygen.
100
4.3
The Osmium(VIII) – Osmium(VI) Reaction
4.3.1
Literature Review
4.3.1.1 Job’s Method of Continuous Variation[49-54]
Job’s method of continuous variation is a commonly used procedure for the
determination of the composition of complexes in solution.
It is thus necessary to
describe the theory surrounding this experimental procedure in addition to deriving
analytical functions for determining conditional equilibrium constants as well as the
molar extinction coefficients of the various reacting species.
Continuous variation diagrams are of a physical property related to the concentration of
an equilibrium two-component complex against volume fraction (χv) or mole fraction (χm)
of one of the two components. Consider the following reaction:
A+B
where:
Keq
AB
…4.1
A = Osmium(VIII)
B = Osmium(VI)
AB = Os2(VII) Intermediate species
A series of solutions can be prepared such that the sum of the concentration of the
respective components remains constant, while the concentration of the individual
components is continuously varied, i.e.:
[A] + [B] = [T]
…4.2
where [T] = The total concentration of the individual components and is constant
The mole fraction of either component can be calculated through the following
equations:
mole A
mole A + mole B
…4.3
mole B
= 1 - χ (A)
mole A + mole B
…4.4
χ (A) =
χ (B) =
101
The equilibrium concentrations of the species are thus given by:
[ A] eq = [T] × χ (A) - [AB]
…4.5
[B] eq = [T] × (1 - χ (A) ) - [AB]
…4.6
In dilute solutions, the thermodynamic equilibrium constant (Keq) for relation 4.1 can be
approximated by the concentration equilibrium constant, Kc:
K eq ≅ K c =
[AB]
[A]eq [B] eq
…4.7
Substitution of equations 4.5 and 4.6 yields the following equation:
K eq =
([T] χ
[AB]
(A)
- [AB] )([T] × (1 - χ
(A)
) - [AB] )
…4.8
Equation 4.9 is obtained when equation 4.8 is rearranged as a quadratic equation with
respect to [AB]:
( K 1eq + [T]) − ( K 1eq + [T]) 2 − [ 4 × [T] 2 × ( χ ( A ) − χ ( A ) )]
2
[ AB] =
…4.9
2
For the general reaction depicted by relation 4.1, the theoretical absorbance of each
component (A, B and AB) at equilibrium could be calculated using the Beer-Lambert
law, provided that the molar extinction coefficient of each species is known:
Abs (A) = ε (A) × [A]eq × l
…4.10
Abs (B) = ε (B) × [B]eq × l
…4.11
Abs (AB) = ε (AB) × [AB]eq × l
…4.12
o
ε = molar extinction coefficient of the respective absorbing species
o
[A]eq, [B]eq, [AB]eq = equilibrium concentration of the respective absorbing species
o
l = optical path length of the cuvette
102
Since the Beer-Lambert law is additive, the contribution of each species’ theoretical
absorbance (assuming that all three species are considered to be absorbing species in
the wavelength region chosen for this study) to the overall theoretical absorbance is:
Abs (Theo) = Abs (A) + Abs (B) + Abs (AB)
…4.13
The numerical values obtained for Abs (Theo) (for each solution in the series) could be
used to ascertain the validity of the estimated values of the parameters Keq, ε (A), ε (B)
and ε (AB). This is achieved using a non-linear least-squares method, which is based on
the assumption that the best estimate for the values of the parameters are those values
that minimise the sum of the squared deviations between the experimentally observed
absorbance data and the theoretically calculated absorbance data. Essentially, this
implies the minimisation of the sum of the squared deviations between the
experimentally observed absorbance data and the theoretical absorbance data
calculated form the proposed equilibrium model (equation 4.9).
Theoretically, the
function minimum (denoted φ2) is represented by:
ϕ2 ≡
M
∑ [Abs
(Theo) i
- Abs (Obs)i ] 2
…4.14
i =1
o
M = Number of data points
o
Abs (Theo)i = Calculated absorbance (at a single wavelength) for the ith spectral
curve
o
Abs (Obs)i = Observed absorbance (at a single wavelength) for the ith spectral
curve
The values assigned to each parameter should therefore be done in a manner which
result in the value of φ2 reaching a minimum.
To calculate the value of Abs (Theo)i, the values of the parameters Keq, ε (A), ε (B) and ε (AB)
are required, which is (in most cases) unknown.
It is assumed here that all three
species have overlapping absorbances and that the molar extinction coefficient of
species AB cannot be measured directly. However, the molar extinction coefficient of
species A can be estimated by obtaining spectra of free A (i.e. in the absence of
species B). Since the concentration and absorbance of species A is known, the molar
extinction coefficient of species A can be approximated by using the Beer-Lambert law.
103
The molar extinction coefficient of species B can be approximated in an analogous
manner.
The evaluation of Keq and ε (AB) can be achieved through a least-squares method in
combination with an iterative minimum-searching routine. Firstly, an initial estimate of
Keq is made, for which a corresponding value of ε (AB) is calculated using the equation:
∆ε = ε ( AB) - ε ( A) - ε (B)
…4.15
When equation 4.15 holds, equation 4.12 can be written as:
∆Abs = Abs
( obs)
− Abs0
…4.16
where: Abs 0 = [(ε (A) − ε (B) ) × χ (B) + ε (B) ] × [T]
By estimating the initial value of Keq, the corresponding value of ε (AB) that would
minimise φ2 for this initial estimate would be given by:
∆ε = ∑
( ∆Abs ) ×([AB] i )
∑ [AB]
2
…4.17
i
Equation 4.17 was derived by setting the partial derivative of φ2 (equation 4.14) with
respect to ∆ε equal to zero[49 – 54].
From the initial estimate of Keq, the best
corresponding value for ∆ε (and consequently ε (AB)) as well as the corresponding φ2
value can be determined.
After each iteration, the value by which Keq is varied
decreases (since there is a decrease in the value of φ2) and the procedure is stopped
when a change in Keq produces a φ2 value that is smaller than a predetermined value.
104
4.3.1.2 Mole Ratio Titrations
One of the limitations to Job’s method of continuous variation is the number of data
points obtained, being dependent on the number of solutions prepared. Due to this
reason, mole ratio titrations were performed, which has the advantage of obtaining a
greater number of data points. Although not used in this study, it is possible to convert
mole ratio titration data to a Job plot. In this manner, Job plots with many data points
can be obtained and less reagent solution is used[55, 56].
The mole ratio method involves keeping the concentration of one reagent constant while
varying the concentration of the other reagent. The resulting absorbances at specific
wavelengths are plotted against the mole ratio of the two reagents. The shape of the
absorbance curve at a particular wavelength depends on the values of the molar
extinction coefficients of the reagents and complexes, and the value of the equilibrium
constant.
4.3.2
Experimental Procedures
4.3.2.1 Job’s Method of Continuous Variation
A 3.172 × 10-3 mol/L aqueous osmium tetroxide stock solution was prepared in distilled
water using the method outlined in Chapter 2.5.3.
A 2 mol/L sodium hydroxide solution was prepared under a nitrogen atmosphere by
dissolving the appropriate amount of sodium hydroxide pellets in degassed water. This
solution was used to dissolve 0.3501 g of purified K2[OsO2(OH)4] crystals in a 250 mL
volumetric flask.
This osmium(VI) stock solution was stored under a nitrogen
atmosphere in order to prevent the oxidation of osmium(VI) by atmospheric oxygen.
The osmium concentration of this stock was determined by the thiourea method, and
was found to be 3.172 × 10-3 mol/L.
Once the concentrations of the osmium(VI) and osmium(VIII) stock solutions were
established, a series of solutions were prepared in 50 mL volumetric flask where the
total molar osmium concentration was maintained at 3.485 × 10-4 mol/L, while the
105
[osmium(VI)]initial/[osmium(VIII)]initial ratio was varied by mixing different volumes of the
osmium(VI) and osmium(VIII) solutions.
A constant reaction volume and hydroxide
concentration were achieved by diluting each solution in the series with specific
volumes of 6 mol/L sodium hydroxide and distilled water, such that the final hydroxide
concentration throughout the series was 2 mol/L.
Following its preparation, each
solution in the series was allowed to equilibrate for approximately 45 seconds after
which its UV-Vis spectrum was recorded.
The experiment was repeated for a total osmium concentration of 7.000 × 10-4 mol/L.
4.3.2.2 Mole Ratio Titrations
Absorbance measurements for the mole ratio titrations were recorded with a
Metrohm 662 photometer, which was described in Chapter 2.1.2.
A mole ratio titration was performed in which 4.065 mL of a 1.431 × 10-3 mol/L osmium
tetroxide stock solution, 12.602 mL distilled water and 8.333 mL of a 6 mol/L sodium
hydroxide solution was transferred to a reaction vessel, such that the total volume of the
solution was 25 mL.
The final osmium(VIII) concentration of this solution was
2.327 × 10-4 mol/L and the sodium hydroxide concentration was 2 mol/L. This solution
was titrated against a 7.636 × 10-3 mol/L potassium osmate solution prepared in a
2 mol/L sodium hydroxide matrix under nitrogen atmosphere. Delivery of the potassium
osmate solution to the reaction vessel was achieved using a Metrohm 665
titroprocessor. During these titrations, the reaction solution was continuously agitated,
but not vigorously to prevent splashes and air bubbles being trapped in the light path of
the photometer probe.
Following each addition of potassium osmate, the reaction
solution was allowed to equilibrate for 15 seconds, after which its absorbance at 400 nm
was recorded. Subsequent titrations were performed with slightly differing osmium(VIII)
concentrations.
The addition of titrant (in this case, potassium osmate) causes the volume of the
reaction solution to increase.
As a result the concentration of the initial reagent,
osmium(VIII), decreases. However, since both the initial reagent as well as the titrant is
osmium species, the absorbance increased continually. It is desirable to show only the
106
changes in absorbance due to reactions of the reagents, and not due to the effect of
changing volume.
This was achieved by “correcting” the absorbance data.
The
corrected absorbance, A, of the reaction solution was calculated from the observed
absorbance, A0, as follows:
A=
where:
A 0 × Vt
Vi
Vi = initial volume
Vt = total volume of the reaction solution
All the spectra and absorbance curves illustrated in this study were corrected in this
manner, except if stated otherwise.
4.3.3
Computer Software Used for Simulating Mole Ratio Titrations
The SPC-V-MR program was used to simulate mole ratio titrations where the mole
ratios between various reagents change. Different reaction models could be simulated
and formation constants and molar extinction values calculated from the experimental
data. The SPC-V-MR program was written by Dr E. Hosten using Borland Turbo Pascal
V6.0[57]. The program can simulate one or more equilibria. From estimates of the
formation constants of each equilibrium and the total reagent concentrations, the
program uses an iterative Gauss-Newton algorithm to calculate the free reagent
(unreacted) concentrations.
Together with the free reagent concentrations, the
estimated formation constants and the molar extinction values, the program can then
calculate the total absorbance.
By comparing the calculated absorbance with the
experimental absorbance, the program uses another iterative Gauss-Newton algorithm
to calculate better estimates for reagent concentrations, formation constants and molar
extinction values.
These iterative cycles then continue until the changes to all the
constants become statistically insignificant. During all the calculations the change in
volume during the titrations due to the addition of titrant, is taken into account. This
program simulates the following type of equilibria:
aA + bB + cC
β=
[A aB b C c ]
[A] a [B] b [C]c
β
A a Bb C c
107
4.3.4
Results and Discussion
4.3.4.1 Job’s Method of Continuous Variation
Figure 4.5 illustrates the change in the UV-Vis spectra as the [Os(VI)]i / [Os(VIII)]i ratio
was gradually increased at a constant total osmium concentration. The figure illustrates
the presence of at least three absorbing osmium species, based on the observation
that, at wavelengths greater than 320 nm, the absorbance initially increases and then
decreases. An identical trend was established in Chapter 3.
1.4
1.2
[14]
[1]
Absorbance
1.0
0.8
0.6
0.4
0.2
[29]
0.0
230
270
310
350
390
430
470
510
550
590
Wavelength /nm
Figure 4.5: The change in absorbance spectra as a function of increasing [Os(VI)]i / [Os(VIII)]i ratio
at pH 14.3. The spectra denoted [1], [14] and [29] corresponds to the [Os(VI)]i / [Os(VIII)]i ratios
-4
0.03, 0.94 and 30.00 respectively. [Os(VI)] + [Os(VIII)] = 3.485 × 10 mol/L
The possibility that the observed absorbance spectrum, produced by reacting
osmium(VIII) with osmium(VI), was simply due to a combination of the respective
species’ absorbance spectra was excluded experimentally.
Since absorbance is
additive, the sum of the pure osmium(VIII) and osmium(VI) absorbance spectra would
produce an addition spectrum if no reaction occurred.
108
1.4
Os(VIII)
Os(VI)
Os2(VII) Intermediate
1.2
Addition spectrum
Intermediate from MeOH
reduction
Absorbance
1.0
0.8
0.6
0.4
0.2
0.0
245
295
345
395
445
495
545
595
Wavelength /nm
-4
Figure 4.6: The UV-Vis spectra of 3.485 × 10 mol/L OsO4 in a 2 mol/L NaOH matrix;
-4
3.485 × 10 mol/L potassium osmate in a 2 mol/L NaOH matrix; the experimentally observed
spectrum
obtained
from
the
reaction
between
-4
1.799 × 10 mol/L
osmium(VIII)
with
-4
1.686 × 10 mol/L potassium osmate in a 2 mol/L NaOH matrix; the theoretically calculated
addition spectrum between osmium(VIII) and potassium osmate; and a comparison of the
intermediate species’ spectrum obtained by reacting osmium(VIII) with methanol in a 2 mol/L
NaOH matrix.
It is evident from Figure 4.6 that the theoretical addition spectrum does not correspond
with the experimentally observed absorbance spectrum. This implies that the observed
absorbance spectrum is not simply due to a combination of the osmium(VIII) and
osmium(VI) species’ absorbance spectra. For comparison, the absorbance spectrum of
the intermediate species formed during the reaction of osmium(VIII) with methanol is
included in Figure 4.6. It can be seen that these spectra are identical. Thus, it is clearly
observed that the osmium species produced during the reaction of osmium(VIII) with
osmium(VI) is the same osmium species produced during the reduction of osmium(VIII)
by all the organic substrates used in this study.
109
Figure 4.7 illustrates a non-equimolar Job diagram which indicates complex formation
between osmium(VIII) and osmium(VI) in a 2 mol/L sodium hydroxide matrix.
The
relatively sharp point at mole fraction 0.5, as well as the fact that the sides of the curve
is virtually linear, is indicative of a 1:1 complex formation between osmium(VIII) and
osmium(VI). In addition, the sharp point obtained at mole fraction 0.5 would imply a
relatively high equilibrium constant. The formation of a 2:2 complex was negated based
on the fact that the sides of the Job plot for this type of complex would become concave
under the current experimental conditions.
Thus, the Job plot also allows for the
discrimination between the formation of 1:1 and 2:2 complexes.
1.8
[2]
Absorbance at 370nm
1.6
1.4
1.2
1.0
[1]
0.8
0.6
0.4
0.2
0.0
0.2
0.4
Mole fraction
0.6
[Os(VI)]
[Os(VI)] + [Os(VIII)]
0.8
1.0
Figure 4.7: Non-equimolar Job diagram illustrating complex formation between osmium(VIII) and
-4
osmium(VI) in a 2 mol/L NaOH matrix. [Os(VI)] + [Os(VIII)] = [1] 3.485 × 10 mol/L;
-4
[2] 7.000 × 10 mol/L
Figure 4.8 depicts the Job plot of the complex formation between osmium(VIII) and
osmium(VI), which was simulated based on a 1:1 complex formation, as described in
Chapter 4.3.1.1. The proposed 1:1 stoichiometry produces complelling theoretical fits,
as illustrated by Figure 4.8.
110
1.0
Exp Data
Os(VIII)Theo
Os(VI)Theo
Absorbance at 370nm
0.8
Os2(VII)Theo
Theo Fit
0.6
0.4
0.2
0.0
0.0
0.2
0.4
Mole Fraction
0.6
0.8
1.0
[Os(VI)]
[Os(VI)] + [Os(VIII)]
Figure 4.8: Job diagram depicting the complex formation between osmium(VIII) and osmium(VI) in
a 2 mol/L NaOH matrix. The theoretical fits were simulated on a 1:1complexation model.
-4
[Os(VI)] + [Os(VIII)] = 3.485 × 10 mol/L. Symbols = experimental data; Lines = calculated fits.
The theoretical curves shown in Figure 4.8 can be used to correct the Job plot for
absorbance of the uncomplexed reagent at the specified wavelength. The corrected
absorbance is defined as the measured absorbance minus the sum of the absorbance
of the reagents if no complexation had occurred[58].
111
Absorbance at 370nm
1.0
0.8
[1]
0.6
[4]
0.4
[2]
[3]
0.2
0.0
0.0
0.2
0.4
Mole fraction
0.6
0.8
1.0
[Os(VI)]
[Os(VI)]
+
[Os(VIII)]
Figure 4.9: Correcting a Job plot for the absorbance of the reacting components, as reported in
[58]
literature
. Lines [2] and [3] is subtracted from plot 1 to obtain plot 4. Experimental data are from
-4
an osmium(VIII) – osmium(VI) Job plot at pH 14.3. [Os(VI)] + [Os(VIII)] = 3.485 × 10 mol/L
Graphically, this involves subtracting the line drawn from the 0.00 mole fraction data
point to the 1.0 mole fraction point from the Job plot.
According to the reported literature
[58]
Figure 4.9 illustrates this.
, the assumption made by drawing a line between
the first and last points, is that no complexation occurs. This is incorrect, even with
complexes with a low equilibrium constant. As a result, the “corrected” plot may be a
better graphical presentation of a Job plot, but it becomes unsuitable for the
determination of constants and stoichiometry by non-linear methods of analysis. None
of the Job pots in this study were corrected in this manner.
The analytical functions described in Chapter 4.3.1.1 take into account the absorbance
from uncomplexed reagents by considering the data as the sum of absorbance of the
reagents and complexes, as illustrated in Figure 4.8. Figure 4.8 clearly shows that the
absorbance contribution to the unreacted reagents is non-linear with mole fraction.
112
Table 4.1 shows the constants calculated at several wavelengths by using Job’s method
of continuous variation.
Table 4.1: The molar extinction coefficients and averaged equilibrium constant calculated at
various wavelengths through Job’s method of continuous variation.
Molar extinction coefficient /
3
-1
× 10 L.mol .cm
Wavelength / nm
-1
ε Os(VIII)
ε Os(VI)
ε Os2 (VII)
340
1.080
1.195
6.633
350
1.572
1.076
6.689
360
1.276
0.971
6.488
370
1.184
0.778
5.631
380
1.073
0.651
4.947
390
0.990
0.484
3.903
0.897
0.351
2.882
400
3
-1
Keq / × 10 L.mol
24.37
113
4.3.4.2 Mole Ratio Titrations
The results of several osmium(VIII) versus osmium(VI) mole ratio titrations in a 2 mol/L
sodium hydroxide matrix are illustrated in Figure 4.10.
-4
1.163×10 M
2.327×10-4 M
3.490×10-4 M
Absorbance at 400nm
0.5
0.4
0.3
0.2
0.1
0.0
0
1
2
3
4
Mole Ratio
[Os(VI)]
[Os(VIII)]
5
6
7
Figure 4.10: Absorbance curves from several osmium(VIII) vs. osmium(VI) mole ratio titrations in a
2 mol/L NaOH matrix. Linear regressions were drawn though the linear regions of the curves to
obtain the point of intersect. The initial osmium(VIII) concentration is denoted in the legend.
This figure illustrates a relatively sharp endpoint, indicative of the stability of the
complex. The mole ratio of the endpoint indicates the composition of the complex.
The approximate position of the endpoints can be obtained graphically by looking at the
intersection of the lines drawn from the experimental points before and after the
endpoint, as depicted in Figure 4.10. The average of the intercepts was calculated from
Figure 4.10 and gives a mole ratio value at the endpoint of the titration of 1.07 (i.e. a 1:1
complex). This corresponds to the reaction:
Os(VIII) + Os(VI)
Os2(VII)
114
The program, SPC-V-MR was used to analyse the osmium(VIII) versus osmium(VI)
mole ratio titration data, the results of which is illustrated in Figure 4.11. The simulation
of a single equilibrium with a 1:1 complex results in good absorbance curve fits across
all concentration range investigated. The corresponding species distribution curves are
shown in Figure 4.12.
0.4
Corrected Absorbance at 400nm
3.490×10-4 M
0.3
2.327×10-4 M
0.2
1.163×10-4 M
0.1
0.0
0
1
2
3
Mole Ratio
4
[Os(VI)]
[Os(VIII)]
5
6
7
Figure 4.11: Volume corrected absorbance curves from osmium(VIII) vs. osmium(VI) mole ratio
titrations in a 2 mol/L NaOH matrix. The calculated curves were simulated on a 1:1 complexation
model. The initial osmium(VIII) concentrations are denoted in the figure. Symbols = experimental
data; Lines = calculated fits
115
1.0
Os2(VII)
Os(VIII)
Mole fraction (Os(VIII))
0.8
0.6
0.4
0.2
0.0
0
1
2
3
4
Mole Ratio
5
[Os(VI)]
[Os(VIII)]
6
7
Figure 4.12: Species distribution curves of an osmium(VIII) vs. osmium(VI) titration in a 2 mol/L
-4
NaOH matrix. [Os(VIII)] = 2.327 × 10 mol/L
Table 4.2 shows the constants calculated at 400 nm from mole ratio studies. These
results are compared to that obtained from Job calculations.
Table 4.2: Comparison of the molar extinction coefficients and equilibrium constant obtained from
mole ratio studies and Job diagrams at 400 nm
Mole ratio studies /
Wavelength
/ nm
400
Keq /
3
× 10 L.mol
-1
3
-1
× 10 L.mol .cm
Job diagrams /
-1
3
-1
× 10 L.mol .cm
-1
ε Os(VIII)
ε Os(VI)
ε Os2 (VII)
ε Os(VIII)
ε Os(VI)
ε Os2 (VII)
0.880
0.345
2.840
0.897
0.351
2.882
25.74
24.37
116
4.4
Conclusion
Job method and osmium(VIII) – osmium(VI) mole ratio titrations confirmed that there is
complex formation between osmium(VIII) and osmium(VI) in the ratio 1:1. The molar
extinction coefficients and equilibrium constants obtained from these methods were in
good correlation with each other.
On the basis of results obtained, it is possible to postulate the following reaction
between osmium(VIII) and osmium(VI):
2-
OH
O
O
Os
O
2-
O
HO
Os
+
O
OH
OH
HO
OH
O
Keq
HO
O
Os
HO
O
OH
+ 2OH-
Os
O
O
2-
O
OH
O
Figure 4.13: The formation of the dimeric osmium(VII) species
The formation of the mixed oxidation state dimeric species proceeds with the evolution
of two hydroxide ions.
117
CHAPTER 5
Conclusion
5.1
Determination of Osmium Concentration
The thiourea method for determining osmium was tested exhaustively and proved to be
a consistent method for the assay of osmium. The sample preparation and analysis
method was simple. The osmium-thiourea complex formation occurred rapidly in the
case of osmate ([OsO2(OH)4]2-), perosmate ([OsO4(OH)2]2-) and osmium tetroxide, but
was slow in the case of hexachloroosmium(IV). It was established that a vast excess of
thiourea (at least 4300 times) was required before consistent spectra were obtained.
Using these parameters, it was possible to develop an analysis method to fit all the
criteria. A linear calibration curve was obtained over the osmium concentration range
5.257 × 10-6 to 2.628 × 10-4 mol/L.
5.2
The Osmium(VIII) – Alcohol Reaction
Since the osmium(VIII) – alcohol reactions were conducted in a 2 mol/L sodium
hydroxide matrix, the stability of osmium tetroxide in this matrix was investigated. It was
found that at a pH of 14.3, osmium(VIII) spontaneously reduced over time to form
osmium(VI). The progress curves obtained from this investigation suggested that the
spontaneous reduction of osmium(VIII) in a 2 mol/L sodium hydroxide matrix followed a
distinct two-step process, with at least three absorbing species being present, i.e. the
initial osmium(VIII) species, an osmium(VII) intermediate species and the final
osmium(VI) product.
Various studies have reported to have established the reaction equilibrium of osmium
tetroxide with hydroxide. These studies, having examined the speciation of osmium
tetroxide as a function of pH are in general concurrence. At pH 14.3 it was reported that
two osmium(VIII) species were present, namely the [OsO4(OH)]- and [OsO4(OH)2]2-
118
species, present in a 40%:60% ratio. It has also been reported[16] that an increase in
the hydroxide concentration led to an increase in the rate at which osmium(VIII) was
reduced by alcohol. In a 3 mol/L sodium hydroxide matrix, the two osmium(VIII) species
would be present in the ratio 30% [OsO4(OH)]- to 70% [OsO4(OH)2]2-.
It therefore
seemed that there was a correlation between the concentration of the [OsO4(OH)2]2species and the rate of the reaction. However, this is not necessarily the case. It
should be borne in mind that, at a pH > 14, the [OsO4(OH)2]2- species reduces to form
the [OsO2(OH)4]2- species. This process would become more pronounced at higher
hydroxide concentrations, which could lead to the false correlation that an increase in
the [OsO4(OH)2]2- species’ concentration leads to an increase in the rate of the oxidation
of alcohols by this species.
The results obtained from the reduction of osmium(VIII) by several primary alcohols
suggests that this reaction proceeds via a distinct two-step process, identical to the
trend observed for the spontaneous reduction of osmium(VIII) at pH 14.3. The influence
that the spontaneous reduction of osmium(VIII) has on the kinetics of the
osmium(VIII) - alcohol reaction was found to be negligible, since the rate of the alcohol
reduction of osmium(VIII) is orders of magnitude greater than the reaction conducted in
the absence of organic substrate. The rate at which osmium(VIII) was reduced was
found to increase along the trend methanol < ethanol < propan-1-ol < butan-1-ol.
Two-dimensional Mauser diagrams were used to analyse the kinetic data obtained from
the reduction of osmium(VIII) with alcohol in a hydroxide matrix.
These diagrams
indicated the occurrence of two consecutive reduction reactions, and allowed for the
calculation of the calculation of the postulated osmium(VII) intermediate species.
Kinetic modelling software was used to fit several theoretical models to the
experimentally obtained kinetic data. The model that produced the best theoretical fit
was given by:
Os(VIII) + RCH2OH
Os(VIII) + Os(VI)
k1
k+2
k-2
Os(VI) + RCHO
Os2(VII)
119
This model is mechanistically feasible, in that one molecule of osmium(VIII) reacts with
one molecule of alcohol to exchange two electrons.
In terms of the reaction
mechanism, it was postulated that the reaction is initiated by an O – H bond cleavage,
as illustrated by Figure 3.28.
2-
OH
O
O
O
Os
O
O
O
O
Os
HO
OH
H
H
+ R
Os
HO
O
H
OH
HO
C
OH
O
H
H
C
2-
O
H2O
O
H
O
2-
OH
C
H
OH-
R
R
Figure 3.28: The hydride transfer reaction mechanism – from the associative reaction of the
primary alcohol molecule with the osmium(VIII) centre, leading to the formation of the osmate ion
and the aldehyde
This conclusion is based on the fact that osmium(VIII), being a d0 species, is normally
associated with strong σ- and π-donor ligands, since these ligands form stable
complexes with ions possessing few or no d-electrons. Oxygen would therefore be an
excellent ligand for stabilising the d0 osmium(VIII) ion and the initial association of the
alcohol through the oxygen atom would be far more favourable.
Despite the increasing acidity of the alcohol in the order primary alcohol < methanol, the
rate of osmium(VIII) reduction was found to increase in the order methanol < primary
alcohol. This trend can be explained if the first step in the depicted reaction mechanism
is not considered to be the rate limiting step. The fact that the hydride ion transfer is
considered to be the rate limiting step of this reaction mechanism, infers that the more
stable molecule in the absence of the hydride ion would result in an increase in the
reaction rate. Abstraction of the hydride ion results in the formation of a positive charge
on the α – carbon of the alcohol molecule.
The larger and more polarisable the
substituent alkyl groups attached to the α – carbon, the more electron density would be
shifted toward that transient positive charge, which would result in a lower energy
O
120
transition state. This interpretation elucidates the increase in the reaction rate with the
increasing number of alkyl groups attached to the α – carbon.
5.3
The Osmium(VIII) – Osmium(VI) Complexation
The complexation between osmium(VIII) and osmium(VI) at pH 14.3 was investigated
spectrophotometrically using Job’s method of continuous variation and mole ratio
titrations.
Mole fraction plots and mole ratio titrations at pH 14.3 indicate that only a single 1:1
complex forms at this pH. Using the analytical functions described in Chapter 4.3.1.1
and custom written software, the equilibrium constant and molar extinction coefficients
of the three postulated species were determined. The results of these calculations are
summarised in Table 5.1. In addition, this table compares the data obtained from mole
fraction plots and mole ratio titrations to that obtained from Mauser diagrams and least
squares analysis of the kinetic data obtained from the osmium(VIII) – alcohol reaction.
Table 5.1 illustrates an excellent correlation of the equilibrium constants and molar
extinction coefficients for all the computational methods used.
121
Table 5.1: Comparison of the equilibrium constant and molar extinction coefficients calculated
through several computational methods. MD = Mauser diagrams; LS = Least square analysis;
JD = Job diagrams; MR = Mole ratio titrations
Wavelength
Molar extinction coefficient /
/ nm
× 10 L.mol .cm
3
MD
JD
1.466
1.572
6.957
6.689
ε Os(VI)
1.101
1.076
ε Os(VIII)
1.329
1.276
6.635
6.488
ε Os(VI)
0.966
0.971
ε Os(VIII)
1.199
1.184
6.397
5.631
ε Os(VI)
0.780
0.778
ε Os(VIII)
1.024
1.073
5.499
4.947
ε Os(VI)
0.676
0.651
ε Os(VIII)
0.990
0.990
4.674
3.903
ε Os(VI)
0.479
0.484
ε Os(VIII)
0.859
0.897
0.880
3.104
2.882
2.840
0.348
0.351
0.345
24.67
24.37
25.74
ε Os2 (VII)
ε Os2 (VII)
360
ε Os2 (VII)
370
ε Os2 (VII)
380
ε Os2 (VII)
390
ε Os2 (VII)
400
ε Os(VI)
3
-1
LS
ε Os(VIII)
350
-1
-1
Keq / × 10 L.mol
6.570
6.214
5.641
4.762
3.744
2.834
MR
122
On the basis of the results acquired, it was possible to postulate the following reaction
between osmium(VIII) and osmium(VI):
2-
OH
O
O
Os
O
HO
OH
Os
+
O
OH
2-
O
HO
OH
O
Keq
HO
O
Os
HO
O
OH
+ 2OH-
Os
O
O
2-
O
OH
O
Figure 4.13: The formation of the dimeric osmium(VII) species.
In order to conclusively prove the existence of the dimeric osmium(VII) species, more
rigorous techniques would be required, including: electron spin resonance (ESR)
spectroscopy, mass spectrometry and osmium nuclear magnetic resonance (NMR)
spectroscopy. Osmium NMR would be a particularly problematic technique to employ
under the current set of experimental conditions, since large concentrations (in excess
of 5 × 10-2 mol/L) osmium would be required in order to obtain the required signal
intensity.
123
APPENDIX
Development of the Program GP2
A.1
Introduction
The program GP2 was developed specifically for the geometric analysis of twodimensional Mauser diagrams.
This program allows for the absorbance of data
acquired at each wavelength to be plotted against the absorbance data of subsequent
wavelengths. For instance, the absorbance data at wavelength i is plotted against the
absorbance data acquired at every wavelength from wavelength (i + a) [where a = 1; 2;
3; …] to wavelength j; resulting in a total of [j - (i + 1)] Mauser diagrams being analysed
for wavelength i.
Employing a linear least-squares algorithm, the program fits two
regression lines to user-defined series for each of the Mauser diagrams generated at
wavelength i, in an analogous manner to the diagram depicted in Figure 3.20. The
coordinates for the point of intersect between the regression lines are then calculated
for each of the diagrams, and an average absorbance value is calculated at each
wavelength. The process is repeated for all subsequent wavelengths.
124
2.2
C
2.0
B [2]
1.8
[3]
Absorbance j
1.6
1.4
1.2
1.0
B
0.8
0.6
A
[1]
0.4
0.2
0.0
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
Absorbance i
Figure 3.20: Typical 2-dimensional Mauser diagram for the general reaction A
Figure A 1: Program interface after data selection
1.6
↔B↔C
125
Figure A 1 depicts the user interface of the program GP2 after the data have been
imported as a tab delimited text file. Once the required data was has been imported,
the user can select the data required for the construction of the linear regressions.
DataSet 1 corresponds to the construction of regression [1] depicted in Figure 3.20,
while DataSet 2 corresponds to regression [2].
In certain cases, reaction systems described by two linearly independent reaction steps
also produce Mauser diagrams in which a straight line is obtained in the Mauser space
(i.e. describing a system with a single linearly independent reaction step). Therefore,
the program features an angle filter, which can be varied as required by the user. The
angle filter prevents the inclusion of outliers in the average absorbance values returned
by the program. These outliers typically occur when the wavelengths used to construct
the diagram are so close to each other (e.g. absorbance at i versus absorbance at
(i + 1)) that the absorbance values of the respective wavelengths are indistinguishable
from each other. In essence, the angle filter determines the angle formed at the point at
which the regression lines intersect. If the calculated angle does not fall within a userdefined range, the absorbance determined at that point is excluded and has no
contribution to the final average absorbance returned by the program for that particular
wavelength.
126
A.2
Listing of the Program GP2
The following is the listing of the Visual Studio.Net[54] code from the program GP2 used
for the analysis of the osmium(VIII) – alcohol kinetic data through Mauser diagrams.
Imports System.IO
Imports System.Data
Imports System.Data.OleDb
Imports System.Collections
Public Class Form1
Public myFile As String = ""
Public dTable As DataTable
Public finalTable As DataTable
Public regArray(1, 3) As Double
Public mVal As Double
Public interC As Double
Public mVal2 As Double
Public interC2 As Double
Public tempTable As New DataTable
Public tempTable2 As New DataTable
Public err As Boolean = False
Public finalSet As New DataSet
Public dSet As New DataSet
Public tempSet As New DataSet
Public tempSet2 As New DataSet
Public meanTable As New DataTable
Public meanSet As New DataSet
Public emptyTable As New DataTable
Private Sub OpenFile()
With OpenFileDialog1
.Filter = "TextFiles (*.txt)|*.txt|All Files (*.*)|*.*"
.DefaultExt = "txt"
.InitialDirectory = ""
If .ShowDialog() = Windows.Forms.DialogResult.OK Then
myFile = .FileName()
Else
myFile = ""
End If
'========================================================'
If myFile <> "" Then
Dim detRecord As New System.Text.StringBuilder()
Dim rec As String
Dim recNbr As Integer
Dim myDataTable As New DataTable("myTable")
Dim myDataCol As DataColumn
Dim myDRow As DataRow
Dim myArray As String()
127
Try
recNbr = 0
If File.Exists(myFile) And myFile.Length > 0 Then
Dim srDetails As StreamReader = New StreamReader(myFile)
rec = srDetails.ReadLine()
myArray = rec.Split(ControlChars.Tab)
Dim s As String
For Each s In myArray
myDataCol = New DataColumn
myDataCol.DataType = System.Type.GetType("System.String")
myDataCol.ColumnName = s
myDataTable.Columns.Add(myDataCol)
Next
dSet.Tables.Add(myDataTable)
grid.DataSource = dSet.Tables("myTable").DefaultView
Do Until srDetails.Peek = -1
rec = srDetails.ReadLine()
myArray = rec.Split(ControlChars.Tab)
myDRow = myDataTable.NewRow()
Dim i As Integer = 0
While i < myArray.Length()
myDRow(i) = myArray.GetValue(i)
i += 1
End While
myDataTable.Rows.Add(myDRow)
grid.Item(0, recNbr).Value = recNbr + 1
recNbr += 1
Loop
srDetails.Close()
Else
MessageBox.Show("File does not exist")
End If
Catch ex As Exception
MessageBox.Show(ex.ToString)
End Try
dTable = myDataTable
End If
End With
End Sub
Private Sub btnExit_Click(ByVal sender As System.Object, ByVal e As System.EventArgs) Handles
btnExit.Click
Me.Close()
End Sub
Private Sub btnSelectD1_Click(ByVal sender As System.Object, ByVal e As System.EventArgs)
Handles btnSelectD1.Click
Dim n, i As Integer
128
Dim a, b As Integer
a = System.Convert.ToInt32(txb1.Text.ToString)
b = System.Convert.ToInt32(txb2.Text.ToString)
i = grid.CurrentCell.ColumnIndex
Dim dRow As DataRow
Dim dCol As DataColumn
Dim dCol2 As DataColumn
Dim dCol3 As DataColumn
For n = 0 To dSet.Tables.Item(0).Columns.Count - 1
dCol = New DataColumn()
dCol2 = New DataColumn()
dCol3 = New DataColumn()
dCol.DataType = System.Type.GetType("System.String")
dCol2.DataType = System.Type.GetType("System.String")
dCol3.DataType = System.Type.GetType("System.String")
dCol.ColumnName = dSet.Tables.Item(0).Columns.Item(n).ColumnName
dCol2.ColumnName = dSet.Tables.Item(0).Columns.Item(n).ColumnName
dCol3.ColumnName = dSet.Tables.Item(0).Columns.Item(n).ColumnName
tempTable.Columns.Add(dCol)
tempTable2.Columns.Add(dCol2)
meanTable.Columns.Add(dCol3)
Next
tempSet.Tables.Add(tempTable)
tempSet2.Tables.Add(tempTable2)
meanSet.Tables.Add(meanTable)
dRow = meanTable.NewRow()
meanTable.Rows.Add(dRow)
Dim k As Integer
For n = a - 1 To b - 1
dRow = tempTable.NewRow()
For k = 0 To dSet.Tables.Item(0).Columns.Count - 1
dRow(k) = dSet.Tables(0).Rows(n).Item(k)
Next
tempTable.Rows.Add(dRow)
Next
gridTemp.DataSource = tempSet.Tables(0).DefaultView
grid2.DataSource = tempSet2.Tables(0).DefaultView
gridMean.DataSource = meanSet.Tables(0).DefaultView
End Sub
Private Sub btnSelectD2_Click(ByVal sender As System.Object, ByVal e As System.EventArgs)
Handles btnSelectD2.Click
Dim n, i As Integer
Dim a, b As Integer
a = System.Convert.ToInt32(txb3.Text.ToString)
b = System.Convert.ToInt32(txb4.Text.ToString)
i = grid.CurrentCell.ColumnIndex
Dim dRow As DataRow
Dim k As Integer
For n = a - 1 To b - 1
dRow = tempTable2.NewRow()
For k = 0 To dSet.Tables.Item(0).Columns.Count - 1
dRow(k) = dSet.Tables(0).Rows(n).Item(k)
Next
tempTable2.Rows.Add(dRow)
Next
End Sub
129
Private Sub OpenToolStripMenuItem1_Click(ByVal sender
System.EventArgs) Handles OpenToolStripMenuItem1.Click
OpenFile()
End Sub
As
System.Object,
ByVal
e
As
Private Sub btnClearD1_Click(ByVal sender As System.Object, ByVal e As System.EventArgs)
Handles btnClearD1.Click
End Sub
Private Sub btnClearD2_Click(ByVal sender As System.Object, ByVal e As System.EventArgs)
Handles btnClearD2.Click
End Sub
Public arrayBase(tempTable.Rows.Count) As Double
Public arrayTemp(tempTable.Rows.Count) As Double
Public arrayBase2(tempTable2.Rows.Count) As Double
Public arrayTemp2(tempTable2.Rows.Count) As Double
Public arrayAns(tempTable.Columns.Count) As Double
Public ansCount As Integer = 0
Public arrayAll(0) As Double
Public arrayAllTemp(0) As Double
Private Sub btnCalcMean_Click(ByVal sender As System.Object, ByVal e As System.EventArgs)
Handles btnCalcMean.Click
Dim ParrayBase(tempTable.Rows.Count) As Double
Dim ParrayTemp(tempTable.Rows.Count) As Double
Dim ParrayBase2(tempTable2.Rows.Count) As Double
Dim ParrayTemp2(tempTable2.Rows.Count) As Double
Dim ParrayAns(tempTable.Columns.Count - 1) As Double
Dim ParrayAll(dTable.Rows.Count) As Double
Dim ParrayTempAll(dTable.Rows.Count) As Double
'populate base array
Dim n As Integer
'Dim k As Integer = 0
Dim base As Integer
Dim i As Integer
For base = 0 To tempTable.Columns.Count - 1
For n = 0 To dTable.Rows.Count - 1
ParrayAll.SetValue(System.Convert.ToDouble(dTable.Rows(n).Item(base).ToString), n)
Next
arrayAll = ParrayAll
For n = 0 To tempTable.Rows.Count - 1
ParrayBase.SetValue(System.Convert.ToDouble(tempTable.Rows(n).Item(base).ToString), n)
Next
For n = 0 To tempTable2.Rows.Count - 1
ParrayBase2.SetValue(System.Convert.ToDouble(tempTable2.Rows(n).Item(base).ToString),
n)
Next
arrayBase = ParrayBase
arrayBase2 = ParrayBase2
For i = 0 To tempTable.Columns.Count - 1
If i = base Then
Else
For n = 0 To dTable.Rows.Count - 1
130
ParrayTempAll.SetValue(System.Convert.ToDouble(dTable.Rows(n).Item(i).ToString), n)
Next
arrayAllTemp = ParrayTempAll
For n = 0 To tempTable.Rows.Count - 1
ParrayTemp.SetValue(System.Convert.ToDouble(tempTable.Rows(n).Item(i).ToString), n)
Next
For n = 0 To tempTable2.Rows.Count - 1
ParrayTemp2.SetValue(System.Convert.ToDouble(tempTable2.Rows(n).Item(i).ToString),
n)
Next
arrayTemp = ParrayTemp
arrayTemp2 = ParrayTemp2
calculateRegression1()
calculateRegression2()
If
(calcAngle()
<
System.Convert.ToDouble(txbA1.Text)
System.Convert.ToDouble(txbA2.Text)) Then
Else
ParrayAns(ansCount) = calcintercept()
ansCount += 1
End If
End If
Next
Dim sum As Double = 0
Dim p As Integer
For p = 0 To ParrayAns.Length - 1
sum += ParrayAns(p)
Next
ansCount = 0
meanTable.Rows(0).Item(base) = sum / (ParrayAns.Length - 1)
Dim b As Double = calcBiSection(base)
Next
MessageBox.Show("calculation comlete")
End Sub
Public Sub calculateRegression1()
Dim x As Double
Dim y As Double
Dim sumP As Double = 0
Dim sumX As Double = 0
Dim sumY As Double = 0
Dim sumSq As Double = 0
Dim totalSumSq As Double
Dim n As Integer
Dim m As Double
Dim b As Double
Dim count As Integer = 0
n = arrayBase.Length - 1
For count = 0 To arrayBase.Length - 2
x = arrayBase(count)
y = arrayTemp(count)
sumP += x * y
sumX += x
sumY += y
sumSq += x * x
Or
calcAngle()
>
131
Next
totalSumSq = sumX * sumX
'===================================
'calculate slope
m = ((n * sumP) - (sumX * sumY)) / ((n * sumSq) - totalSumSq)
'round
m = Math.Round(m, 4)
mVal = m
'calculate intercept
b = (sumY - (m * sumX)) / n
'round
b = Math.Round(b, 4)
interC = b
End Sub
Public Sub calculateRegression2()
Dim x As Double
Dim y As Double
Dim sumP As Double = 0
Dim sumX As Double = 0
Dim sumY As Double = 0
Dim sumSq As Double = 0
Dim totalSumSq As Double
Dim n As Integer
Dim m As Double
Dim b As Double
Dim count As Integer = 0
n = arrayBase2.Length - 1
For count = 0 To arrayBase2.Length - 2
x = arrayBase2(count)
y = arrayTemp2(count)
sumP += x * y
sumX += x
sumY += y
sumSq += x * x
Next
totalSumSq = sumX * sumX
'===================================
'calculate slope
m = ((n * sumP) - (sumX * sumY)) / ((n * sumSq) - totalSumSq)
m = Math.Round(m, 4)
mVal2 = m
'calculate intercept
b = (sumY - (m * sumX)) / n
b = Math.Round(b, 4)
interC2 = b
End Sub
Public Function calcintercept() As Double
Dim x As Double
Dim b As Double
Dim ans As Double
x = mval - mval2
b = interc2 - interc
If x <> 0 Then
ans = b / x
132
Else
ans = 1000
err = True
End If
Return ans
End Function
Private Sub CloseToolStripMenuItem_Click(ByVal sender
System.EventArgs) Handles CloseToolStripMenuItem.Click
As
System.Object,
ByVal
e
As
ByVal
e
As
End Sub
Private Sub ExitToolStripMenuItem_Click(ByVal sender
System.EventArgs) Handles ExitToolStripMenuItem.Click
Me.Close()
End Sub
As
System.Object,
Public angX1, angX2, angX3 As Double
Public angY1, angY2, angY3 As Double
Public d1, d2, d3 As Double
Private Function calcAngle() As Double
angX1 = arrayBase(1)
angX2 = arrayBase2(arrayBase2.Length - 2)
angY1 = mVal * angX1 + interC
angY2 = mVal2 * angX2 + interC2
angX3 = calcintercept()
angY3 = mVal * angX3 + interC
d1 = Math.Sqrt(((angX1 - angX2) * (angX1 - angX2)) + ((angY1 - angY2) * (angY1 - angY2)))
d2 = Math.Sqrt(((angX3 - angX2) * (angX3 - angX2)) + ((angY3 - angY2) * (angY3 - angY2)))
d3 = Math.Sqrt(((angX1 - angX3) * (angX1 - angX3)) + ((angY1 - angY3) * (angY1 - angY3)))
Dim f, f4 As Double
Dim t1, t2 As Double
t1 = d3 * d3 + d2 * d2 - d1 * d1
t2 = 2 * d3 * d2
''f = Math.Cos((t1) / (t2))
''f3 = Math.Cosh(t1 / t2)
f4 = Math.Acos(t1 / t2)
f = f4 * (180 / Math.PI)
''f2 = 1 / f
If angX3 = 1000 Then
Return System.Convert.ToDouble(txbA1.Text) + 0.0001
Else
Return f
End If
End Function
Private Function calcTrapesium(ByVal Count As Double, ByVal startX As Double, ByVal startY As
Double) As Double
Dim h, b1, b2 As Double
Dim initX As Double = startX 'arrayAll(0)
Dim initY As Double = startY 'arrayAllTemp(0)
Dim Sum As Double = 0
Dim finY, finX As Double
133
Dim PrevX As Double = arrayAll(0)
Dim PrevY As Double = arrayAllTemp(0)
'calc base line
finX = finalArray(Count)
finY = mVal2 * finX + interC2
If (initX < finX) Then
If (initY > finY) Then
Dim grad As Double = ((initY - finY) / (initX - finalArray(Count)))
b1 = 0
Dim i As Integer = 0
For i = 1 To arrayAll.Length - 2
Dim nextX As Double = arrayAll(i)
Dim nextY As Double = arrayAllTemp(i)
Dim C As Double = nextY - (nextX * ((1 / grad) * -1))
'Dim C As Double = nextY - (nextX * 6)
Dim crossX As Double
Dim ans As Double
crossX = grad - ((1 / grad) * -1)
Dim tempVal As Double = initY - grad * initX
'crossX = grad - 6
Dim b As Double = C - tempVal '50 'arrayAllTemp(0)
If crossX <> 0 Then
ans = b / crossX
Else
ans = 1000
'err = True
End If
h = Math.Sqrt(Math.Pow((PrevX - ans), 2) + Math.Pow((PrevY - (((1 / grad) * -1) * ans + C)),
2))
b2 = Math.Sqrt(Math.Pow((nextX - ans), 2) + Math.Pow((nextY - (((1 / grad) * -1) * ans + C)),
2))
Sum = Sum + (0.5 * h * (b1 + b2))
b1 = b2
PrevX = ans
PrevY = ((1 / grad) * -1) * ans + C
Next
Else
initY = finY
initX = finX
finY = startY '[arrayTemp(0)
finX = startX 'arrayBase(0)
Dim grad As Double = ((initY - finY) / (initX - finalArray(Count)))
Dim tempVal As Double = initY - grad * initX
b1 = 0
Dim i As Integer = 0
For i = 1 To arrayAll.Length - 2
Dim nextX As Double = arrayAll(i)
Dim nextY As Double = arrayAllTemp(i)
Dim C As Double = nextY - (nextX * ((1 / grad) * -1))
'Dim C As Double = nextY - (nextX * 6)
Dim crossX As Double
134
Dim ans As Double
crossX = grad - ((1 / grad) * -1)
'crossX = grad - 6
Dim b As Double = C - arrayAllTemp(0)
If crossX <> 0 Then
ans = b / crossX
Else
ans = 1000
'err = True
End If
h = Math.Sqrt(Math.Pow((PrevX - ans), 2) + Math.Pow((PrevY - (((1 / grad) * -1) * ans + C)),
2))
b2 = Math.Sqrt(Math.Pow((nextX - ans), 2) + Math.Pow((nextY - (((1 / grad) * -1) * ans + C)),
2))
Sum = Sum + (0.5 * h * (b1 + b2))
b1 = b2
PrevX = ans
PrevY = ((1 / grad) * -1) * ans + C
Next
End If
Else
If (initY > finY) Then
'Next
Else
initY = finY
initX = finX
finY = startY 'arrayAllTemp(0)
finX = startX 'arrayAll(0)
Dim grad As Double = ((initY - finY) / (initX - finX))
Dim tempVal As Double = initY - grad * initX
b1 = 0
Dim i As Integer = 0
PrevX = initX
PrevY = initY
For i = arrayAll.Length - 3 To 0 Step -1
Dim nextX As Double = arrayAll(i)
Dim nextY As Double = arrayAllTemp(i)
Dim C As Double = nextY - (nextX * ((1 / grad) * -1))
Dim crossX As Double
Dim ans As Double
crossX = grad - ((1 / grad) * -1)
'crossX = grad - 6
Dim b As Double = C - tempVal '160 'arrayAllTemp(arrayAll.Length - 2)
If crossX <> 0 Then
ans = b / crossX
Else
ans = 1000
'err = True
End If
h = Math.Sqrt(Math.Pow((PrevX - ans), 2) + Math.Pow((PrevY - (((1 / grad) * -1) * ans + C)),
2))
b2 = Math.Sqrt(Math.Pow((nextX - ans), 2) + Math.Pow((nextY - (((1 / grad) * -1) * ans + C)),
2))
Sum = Sum + (0.5 * h * (b1 + b2))
135
b1 = b2
PrevX = ans
PrevY = ((1 / grad) * -1) * ans + C
Next
End If
End If
Return Sum
End Function
End Class
136
REFERENCES
1)
Griffith, WP. In: The Chemistry of the Rarer Platinum Metals (Os, Ru, Ir & Rh).
Chapter 3. 1967, Interscience.
2)
Brunot, FR. J. Ind. Hyg. & Tox. 1933, 136-143.
3)
McLaughlin, AIG, Milton, R and Perry, KMA. British J of Ind. Med. 1946, 183186.
4)
http://www.webelements.com/webelements/elements/text/Os/
5)
Griffith, W.P. Platinum Metals Review. 2004, 48(4), 182-189.
6)
Griffith W.P., Osmium. In: Comprehensive Coordination Chemistry. The
synthesis, reactions, properties and applications of coordination compounds.
1987. Vol. 4. Ed. G. Wilkinson. Pergamon Press, Oxford.
7)
Millipore Simplicity Water Purification System: Operating and Maintenance
Manual
8)
Sauerbrunn, RD and Sandell, EB. J. Am. Chem. Soc. 1953, 75, 3554
9)
Ayres, GH and Wells, WN. Anal. Chem., 1950, 22, 317-320
10)
Cristiani, F, Devillanova, FA, Diaz, A and Verani, G. Inorg. Chim. Acta, 1981,
50, 251-255
11)
Chugaev, L and Gritzmann, EZ. Anorg. Allg. Chem. 1928, 172, 213
12)
Suzuki, T, Miyada, M, Ohta, K, Kaneco, S and Mizuno, T. Microchim. Acta,
1998, 129, 259-263
13)
Anderson, LH and Yost, DM. J. Am. Chem. Soc., 1938, 60, 1822-1825
14)
Wells, EJ, Jordan, AD, Alderdice, DS and Ross, IG. Aust. J. Chem., 1967, 20,
2315-2322
15)
Roebber, JL, Wiener, RN, Russel, CA. J. Phys. Chem., 1974, 60, 3166-3173
16)
McFadzean, BJ. PhD Dissertation, 2008, NMMU, South Africa
17)
The Royal Swedish Academy of Sciences. Advanced information on the Nobel
Prize in Chemistry 2001.
http://nobelprize.org/nobel_prizes/chemistry/laureates/2001/chemadv.pdf.
18)
Singh, HS, Singh, SP, Singh, SM, Singh, RK and Sisodia, AK. J. Phys. Chem.
1975, 79(18), 1920-1924.
137
19)
Singh, NP, Singh, VN and Singh, MP. Aust. J. Chem. 1968, 21, 2913-8.
20)
Singh, NP, Singh, VN, Singh, HS, Singh, MP. Aust. J. Chem. 1970, 23, 921-8.
21)
Singh, VN, Singh, HS and Saxena, BBL. J. Am. Chem. Soc. 1969, 91(10),
2643-8
22)
Singh, B, Singh, BB and Singh, RP. J Inorg. Nucl. Chem. 1981, 43, 1283-6.
23)
Singh, H.S. Oxidation of Organic Compounds with Osmium Tetroxide. In:
Organic synthesis by oxidation with metal compounds. 1986. Ed. W.J. Mijs &
C.R.H. de Jonge. Plenum Press, New York.
24)
Lay,PA and Harman, WD. Recent advances in inorganic chemistry. 1991, 37,
219-379
25)
Criegee, R. Liebigs Ann. Chem. 1936, 522.
26)
http://www.acros.com/_Rainbow/pdf/AO_Oxidation_OSMIUM_DEF.pdf.
27)
McMurray, J. Organic Chemistry – 4th Ed. 1996. Brooks/Cole Publishing
Company, Pacific Grove, USA.
28)
Veerasomaiah, P, Reddy, KB, Sethuram, B and Navaneeth Rao, T. J Indian
Chem. Soc. 1989, 66, 755-8.
29)
Subbaraman, LR, Subbaraman, J and Behrman, EJ. Inorganic Chemistry. 1972,
11(11), 2621-7.
30)
Sharpless, KB, Teranishi, AY and Backvall, JE. J. Am. Chem. Soc. 1976, 99(9),
3120-7.
31)
Subbaraman, LR, Subbaraman, J and Behrman, EJ. J. Org. Chem. 1973, 38(8),
1499-1504.
32)
Westheimer, FH and Watanaba, W. J. Chem. Phys. 1949, 17, 61
33)
Jordan, RB. Reaction mechanisms of inorganic and organometallic systems.
2nd ed. 1998. Oxford University Press, Oxford.
34)
Rankin, KN, Qing Liu, JH, Yee, H, Nazih, A, Noureldin, A and Lee, DG.
Tetrahedron Letters. 1998, 39, 1095-1098.
35)
Dehestani, A, Lam, WH, Hrovat, DA, Davidson, ER, Borden, WT, Mayer, JM.
J. Am. Chem. Soc., 2005, 127, 3423-3432
36)
Cotton, FA and Wilkinson, G. Advanced Inorganic Chemistry. 4th Ed. 1980,
Wiley-Interscience, New York.
37)
Uma, KV and Mayanna, SM. Journal of Catalysis. 1980, 61, 165-9.
138
38)
Polster, J and Mauser, H. Talanta, 1992, 39 (10), 1355-1359
39)
Polster, J and Dithmar, H. Phys. Chem. Chem. Phys. 2001, 3, 993-991
40)
Polster, J. Chemical Physics, 1999, 240, 331-351
41)
Polster, J. Phys. Chem. Chem. Phys. 1999, 1, 4791-4795
42)
Polster, J. Chemical Physics. 2001, 263, 69-81
43)
Polster, J and Dithmar, H. Chemical Physics, 2002, 283, 473-480
44)
Geswindt, TE, Gerber, WJ, Rohwer, HE and Hosten, EC. A kinetic approach to
determine the activity coefficients of Rhodium(III) species in an HCl matrix,
Unpublished work, 2006
45)
Gerber, WJ. Rhodium(III) Speciation: A Chromatographic Study with ICP-MS,
PhD dissertation, NMMU, Port Elizabeth, 2006
46)
Burden, RL and Faires, JD. 6th Ed, Brookes/Cole Publishing Company, 1997
47)
Galbacs, ZM, Zsednai, A and Csányi, LJ. Transition metal chemistry. 1983, 8,
328-332.
48)
Mouchel, B and Bremard, CJ. Chem. Research (S). 1978, 312-313.
49)
Bruneau, E, Lavabre, D, Levy, G and Micheau, JC. J. Chem. Educ., 1992, 69
(10), 833-837
50)
Gil, VMS and Oliveira, NC. J. Chem. Educ., 1990, 67 (6), 473-478
51)
Hill, ZD and MacCarthy, P. J. Chem. Educ., 1986, 63, 162-167
52)
Long, JR and Drago, RS. J. Chem. Educ., 1982, 59 (12), 1037-1039
53)
MacCarthy, P. Anal. Chem., 1978, 50 (14), 2165
54)
Likussar, W and Boltz, DF. Anal. Chem., 1971, 43 (10), 1265-1272
55)
Momoki, K, Sekino, J, Sato, H, Yamaguchi, N. Anal. Chem., 1969, 41 (10)
1286-1299
56)
Inczèdy, J. Analytical Applications of Complex Equilibria, Ellis Horwood,
Chichester, 1976
57)
Hosten, EC. Complexing Interactions with Arsenazo(III), PhD dissertation,
NMMU, Port Elizabeth, 1996
58)
Klausen, KS, Langmyhr, F. J., Anal. Chim. Acta., 1963, 28, 335-340
59)
Beaufils, JP. Bull. Soc Chim, 1969, 4, 1066
60)
Beaufils, JP and Coussemant, F. Bull. Soc Chim., 1968, 1, 27
139
61)
Doke, ER, Satzinger, JW, Williams, SR and Douglas, DE. Object-Orientated
Application Development Using Microsoft Visual Bascic.NET, 2003, Course
Technology