* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Types of Chemical Reactions - Celebrity Examples
Inorganic chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Water splitting wikipedia , lookup
Asymmetric induction wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Organic chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Electrolysis of water wikipedia , lookup
Discodermolide wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Process chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Rate equation wikipedia , lookup
Transition state theory wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Click chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Chemical reaction wikipedia , lookup
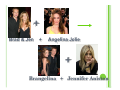
CHEMICAL REACTIONS INDICATORS OF A REACTION | Usually accompanied by easily observed physical effects: y Emission of heat and y Giving off light y Formation of a precipitate y Formation of a gas y Color change TYPES OF CHEMICAL REACTIONS There are 5 basic reaction types: | y y y y y Synthesis Decomposition Single Replacement Double Replacement Combustion SYNTHESIS REACTIONS | Occurs when two substances (generally 2 different elements) combine and form a single new compound y General Formula: A + B AB Kind of like a celebrity marriage… + = Tomkat Tom Cruise Katie Holmes + = Bennifer Ben Affleck Jennifer Garner | Formation of a precipitate is an indication that a combination reaction has taken place y Example: 2 Mg (s) + O2 (g) Magnesium 2 K (s) Potassium Oxygen + Cl2 (g) Chlorine 2 MgO (s) Magnesium Oxide 2 KCl (s) Potassium Chloride DECOMPOSITION REACTIONS |A single compound breaks down into two or more simpler products or elements. y General Formula AB A + B Kind of like a celebrity divorce/breakup… 2 Taylor = + Taylor Lautner Taylor Swift Demton = + Ashton Kutcher Demi Moore | Usually require energy in the form of heat, light, or electricity in order to occur y Example: 2 H2O (l) H2CO3 (aq) electricity heat 2 H2 (g) + O2 (g) 2 H2O (l) + CO2 (g) Decomposition is the OPPOSITE reaction of Synthesis! SINGLE-REPLACEMENT REACTION | Occurs when one element replaces another in a compound. y General Formula: A + BC BA + C In Hollywood, this usually takes place when you star in a movie with an extremely good looking costar… + Brad & Jen + Angelina Jolie + Brangelina + Jennifer Aniston | Generally occur when a ionic compound comes in contact with a more reactive metal or more reactive halogen. y Example: 2 K (s) + 2 H2O (l) 2 KOH (aq) + 2 HCl (aq) + F2 (g) 2 HF (aq) + H2 (g) Cl2 (g) DOUBLE-REPLACEMENT REACTION | Parts of two compounds switch places to form two new compounds y General Formula AB + CD AD + CB Think Hollywood spouse swap… + Justin Timberlake & Gerard Bulter & Cameron Diaz + Jessica Biel + Justin Timberlake & + Gerard Bulter & Jessica Biel Cameron Diaz | Generally take place in aqueous solution | Often produce a precipitate, gas, or molecular compound (H2O) y Example: Na2S (aq) + Cd(NO3)2 (aq) CdS (s) + 2 NaNO3 (aq) 2 NaCN + H2SO4 (aq) 2 HCN (g) + Na2SO4 (aq) COMBUSTION REACTIONS | An element or compound reacts with oxygen gas (O2) | Often produces energy in the form of heat and light | Combustion of a hydrocarbon chain produces water and carbon dioxide y Example: 2 C4H8 (l) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) MAIN CONCEPTS Synthesis y TWO (or more) reactants combine to form ONE product | Decomposition y ONE reactant yields TWO (or more) products | Single-Replacement y ONE element and ONE compound form ONE different element and ONE new compound. | Double-Replacement y TWO reactants yield TWO new products | Combustion y Reaction with oxygen gas and ALWAYS forms water and carbon dioxide |