* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heart failure
Saturated fat and cardiovascular disease wikipedia , lookup
Cardiovascular disease wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Electrocardiography wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Heart failure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Heart failure
• Heart failure (also called congestive heart
failure, or CHF) is a frequent end point of many
of the conditions
• In the United States alone, CHF affects nearly 5
million individuals annually, necessitating >1
million hospitalizations, and contributes to death
of 300,000 patients a year.
• Most heart failure is the consequence of
systolic dysfunction, the progressive
deterioration of myocardial contractile
function.
Causes of CHF
• 1-ischemic heart disease
• 2-hypertension.
-in 20% to 50% of patients the heart
contracts normally but relaxation is
abnormal ("diastolic" failure ).
-the patients with "diastolic" failure are
generally older and more likely to be
female with hypertension or diabetes
mellitus.
• 3-valve failure (e.g., endocarditis)
• 4-normal hearts suddenly burdened
with an abnormal load (e.g., fluid or
pressure overload).
• 5-acute blood loss
• As a compansation the heart dilates the
ventricular wall tension increases which
increases the oxygen requirements of an
already compromised myocardium.
• With time, the failing myocardium is no
longer able to propel sufficient blood to
meet the needs of the body, even at rest.
• At this point, patients enter a phase
termed decompensated heart failure
•
•
•
•
Types of Heart failure:
1- predominantly the left side
2- predominantly the right side
3- both sides of the heart.
• The most common causes of left-sided
cardiac failure are:
• (1) IHD (ischemic heart disease)
• (2) systemic hypertension
• (3) mitral or aortic valve disease
• (4) primary diseases of the
myocardium.
• The most common cause of right-sided heart
failure is:
• 1-left ventricular failure, with its associated
pulmonary congestion and elevation in pulmonary
arterial pressure.
• 2-Right-sided failure can also occur in the absence
of left-sided heart failure in patients with intrinsic
diseases of the lung parenchyma and/or
pulmonary vasculature (cor pulmonale)
cor pulmonale can be caused by :
a. primary pulmonic or tricuspid valve
disease.
b. congenital heart diseases, i.e., left-toright shunts
Clinical manifestations
• Left-Sided Heart Failure
1-Dyspnea (breathlessness) is usually
the earliest and most significant
complaint of patients in left-sided
heart failure;
2-cough is also a common
accompaniment of left heart failure
due to fluid transudation into
airspaces.
3-orthopnea (dyspnea when recumbent)
-This occurs because of increased venous
return from the lower extremities and by
elevation of the diaphragm when in the
supine position.
-Orthopnea is typically relieved by sitting
or standing, so that such patients usually
sleep while sitting upright.
• 4-Paroxysmal nocturnal dyspnea is a
particularly dramatic form of
breathlessness awakening patients from
sleep with attacks of extreme dyspnea
bordering on suffocation.
5-cardiomegaly (enlarged heart )
6-tachycardia (increase heart rate )
7-third heart sound (S3), and fine rales at the
lung bases, produced by respirations through
edematous pulmonary alveoli.
8-mitral regurgitation and a systolic
murmur.
9-atrial fibrillation irregular heartbeat.
Clinical manifestations
• Right-Sided Heart Failure
1-systemic and portal venous congestion
2-hepatic and splenic enlargement
3-peripheral edema
4-pleural effusion
5-ascites
6-cyanosis and acidosis
ISCHEMIC HEART DISEASE
(IHD)
• IHD is also frequently called coronary artery disease
(CAD)
• IHD is a generic designation for a group of related
syndromes resulting from myocardial ischemiaan imbalance between cardiac blood supply
(perfusion) and myocardial oxygen demand.
• ischemia can result from:
• 1- increased demand (e.g., tachycardia
or hypertension
• 2- diminished oxygen-carrying capacity
(e.g., anemia, carbon monoxide
poisoning),
• 3- reduction in coronary blood flow
caused by obstructive atherosclerotic
disease
• There are four basic clinical syndromes
of IHD:
• 1-Angina pectoris
• the ischemia causes pain but is insufficient
to lead to death of myocardium
Types of angina :
1-stable angina (occurring reliably after
certain levels of exertion)
2-variant angina or Prinzmetal angina (
due to vessel spasm )
3-Unstable angina
occurring with progressively less exertion
or even at rest.
• 2-Acute myocardial infarction (MI)
• the severity or duration of ischemia is enough to
cause cardiac muscle death
• 3-Chronic IHD
progressive cardiac decompensation (heart
failure) following MI
• 4-Sudden cardiac death (SCD)
can result from a lethal arrhythmia following
myocardial ischemia.
Epidemiology
• Nearly 500,000 Americans die of IHD
annually
• After peaking in 1963, the overall death
rate from IHD has fallen in the United
States by approximately 50%.
• The decline can be attributed largely to the
recognition of cardiac risk factors.
• Risk factors:
1- smoking.
2- hypertension
3- diabetes.
4- lowering cholesterol levels.
Pathogenesis
• atherosclerotic occlusion of coronary
arteries and new superimposed
thrombosis and/or vasospasm
-lesion obstructing 70% to 75% or more of a
vessel lumen-so-called critical stenosis
→ angina only in the setting of increased
demand
-a fixed 90% stenosis can lead to
inadequate coronary blood flow even at
rest.
- Chronic coronary occlusion
when a coronary artery develops atherosclerotic
occlusion at a sufficiently slow rate, it may be
able to stimulate collateral blood flow from other
major epicardial vessels → protection against MI
even in the setting of a complete vascular
occlusion.
- acute coronary occlusions
cannot spontaneously recruit collateral flow and
will result in infarction
Clinical Features
• 1-severe, crushing substernal chest pain
• 2- discomfort that can radiate to the neck,
jaw, epigastrium, or left arm.
• In contrast to the pain of angina pectoris,
the pain of an MI typically lasts from 20
minutes to several hours and is not
significantly relieved by nitroglycerin or rest.
• 3- MIs can be entirely asymptomatic in
10% to 15% of the cases (silent
infarcts).
• "silent" infarcts are particularly
common in patients with:
• 1- underlying diabetes mellitus (with
peripheral neuropathies)
• 2- in the elderly.
• 4- the pulse is generally rapid and weak
• 5- patients can be diaphoretic and
nauseated particularly with posteriorwall MIs.
• 6- dyspnea is common and is caused
by impaired myocardial contractility
and dysfunction of the mitral valve
apparatus, with resultant pulmonary
congestion and edema.
• 7-With massive MIs (>40% of the left
ventricle) cardiogenic shock develops.
Angina Pectoris
• Angina pectoris is intermittent chest pain caused by
transient, reversible myocardial ischemia. There are
three variants:
• 1-Typical or stable angina
-is episodic chest pain associated with exertion or some
other form of increased myocardial oxygen demand
(e.g., tachycardia or hypertension due to fever, anxiety,
fear).
-the pain is classically described as a crushing or
squeezing substernal sensation,
-the pain can radiate down the left arm or to the left jaw
(referred pain).
- Stable angina pectoris is usually
associated with a fixed atherosclerotic
narrowing (≥75%) of one or more coronary
arteries.
- With this degree of critical stenosis, the
myocardial oxygen supply may be
sufficient under basal conditions but
cannot be adequately augmented to meet
any increased requirements
- The pain is usually relieved by rest
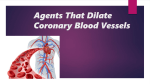
(reducing demand) or by administering
agents such as nitroglycerin;
- such drugs cause peripheral vasodilation
and thus reduce venous blood delivered to
the heart → reducing cardiac work.
- in larger doses, nitroglycerin also
increases blood supply to the myocardium
by direct coronary vasodilation
• 2-Prinzmetal, or variant angina
• Is angina occurring at rest due to coronary artery
spasm.
• Although such spasms typically occur on or near
an existing atherosclerotic plaque, completely
normal vessels can be affected.
• The etiology is not clear.
• Prinzmetal angina typically responds promptly to
the administration of vasodilators such as
nitroglycerin or calcium channel blockers.
• 3-Unstable angina (also called
crescendo angina)
- is characterized by increasing frequency of
pain, precipitated by progressively less
exertion.
- the episodes also tend to be more intense
and longer lasting than stable angina.
- unstable angina is associated with plaque
disruption and superimposed partial
thrombosis, distal embolization of the
thrombus, and/or vasospasm.
- Unstable angina is the harbinger of more
serious, potentially irreversible ischemia
(due to complete luminal occlusion by
thrombus) and is therefore sometimes
called pre-infarction angina.
Myocardial Infarction
• MI, popularly called heart attack,
• is necrosis of heart muscle resulting from ischemia.
• Roughly 1.5 million people in the United States
suffer an MI every year.
• 33-50% die-half before they can reach the hospital.
• Lethal arrhythmia Sudden Cardiac Death
• Arrhythmias are caused by electrical abnormalities
of the ischemic myocardium and conduction system.
• The major underlying cause of IHD is
atherosclerosis and therefore the
frequency of MIs rises progressively
with increasing age and presence of
other risk factors such as
hypertension, smoking, and diabetes
• Acute occlusion of the proximal left
anterior descending (LAD) artery is the
cause of 40-50% of all MIs and typically
results in infarction of the anterior wall of
the left ventricle, the anterior 2/3 of the
ventricular septum, and most of the heart
apex
• Approximately 10% of MIs occur in
people younger than 40 years.
• 45% occur in people younger than age
65.
• Blacks and whites are equally affected.
• Men are at significantly greater risk than
women, although the gap progressively
narrows with age.
• In general, women are remarkably protected
against MI during their reproductive years.
• Nevertheless, menopause and declining
estrogen production- is associated with
exacerbation of coronary atherosclerosis
Electrocardiographic(ECG)
abnormalities
• are important markers of MIs; these include
1-changes such as Q waves (indicating transmural
infarcts),
2- ST-segment abnormalities
3-T-wave inversion (representing abnormalities in
myocardial repolarization).
4-Arrhythmias caused by electrical abnormalities of the
ischemic myocardium and conduction system are
common, and indeed, SCD due to a lethal arrhythmia
accounts for the vast majority of deaths occurring
before hospitalization
Laboratory evaluation of MI
• is based on measuring the blood levels of
intracellular macromolecules that leak out of
injured myocardial cells through damaged cell
membranes.
• these molecules include :
1-myoglobin.
2-cardiac troponins T and I (TnT, TnI),
3-creatine kinase (CK, and more specifically the
myocardial-specific isoform, CK-MB),
4- lactate dehydrogenase, and many others.
• Cardiac troponins T and I (TnT, TnI), are the
best markers for acute MI. persistence of
elevated troponin levels for approximately 10
days allows the diagnosis of acute MI long
after CK-MB levels have returned to normal.
• TnI and TnT are not normally detectable in the
circulation.
• After acute MI both troponins become detectable
after 2 to 4 hours and peak at 48 hours.
• The level remains elevated for 7 to 10 days.
• CK-MB is the second best marker after
the cardiac-specific troponins.
• Since various forms of CK are found in
brain, myocardium, and skeletal muscle,
total CK activity is not a reliable marker of
cardiac injury (i.e. it could come from
skeletal muscle injury).
• CK-MB isoform-principally derived from
myocardium but also present at low levels
in skeletal muscle is the more specific
indicator of heart damage.
• CK-MB activity begins to rise within 2-4
hours of MI, peaks at 24-48 hours, and
returns to normal within approximately
72 hours.
• cardiac troponin and CK-MB are equally
sensitive at early stages of an MI.
• persistence of elevated troponin levels for
approximately 10 days allows the diagnosis
of acute MI long after CK-MB levels have
returned to normal.
• With reperfusion, both troponin and CK-MB
peaks occur earlier as a result of washout of
the enzyme from the necrotic tissue .
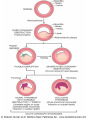
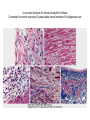
Morphology
1- (<24 hr) coagulative necrosis and
wavy fibers. Necrotic cells are
separated by edema fluid.
2- (2- 3-day) -old infarct Dense
neutrophilic infiltrate
3- (7-10 days) complete removal of
necrotic myocytes by phagocytic
macrophages
4- up to 14 days Granulation tissue
characterized by loose connective
tissue and abundant capillaries.
5- several weeks Healed myocardial
infarct consisting of a dense
collagenous scar.
A-necrosis & edema B- dense neutrophilic infiltrate
C-removal of necrotic myocutes D-granulation tissue formation E-collagenous scar
Consequences and
Complications of MI
• 1-Unfortunately, 50% of the deaths
associated with acute MI occur in
individuals who never reach the hospital.
• patients generally die within 1 hour of
symptom onset-usually as a result of
arrhythmias.
• Extraordinary progress has been made in
patient outcomes subsequent to acute MI.
• Since the 1960s the in-hospital death rate
has declined from approximately 30% to
an overall rate of between 10% and 13%.
• 2- cardiogenic shock.
• occurs in 10-15% of patients after acute
MI, generally with a large infarct (often
>40% of the left ventricle).
• Cardiogenic shock has a nearly 70%
mortality rate and accounts for two-thirds
of in-hospital deaths.
•
•
•
•
•
3-Myocardial rupture
4-Pericarditis.
5-Infarct expansion
6-Ventricular aneurysm
7-Progressive late heart failure
• Complications of myocardial rupture include:
• (1) rupture of the ventricular free wall, with
hemopericardium and cardiac tamponade, which
is usually fatal
• (2) rupture of the ventricular septum, leading to
a new VSD and left-to-right shunt
• (3) papillary muscle rupture, resulting in severe
mitral regurgitation
• Rupture can occur at almost any time after
MI but is most common 3 to 7 days after
infarction.
• It is at this point in the healing process that
lysis of the myocardial connective tissue is
maximal and the granulation tissue has
not deposited sufficient collagenous matrix
to buttress the wall .
• Risk factors for free-wall rupture
include :
• 1-age older than 60 years
• 2-female gender
• 3-pre-existing hypertension
• Factors that prevent rupture are:
• 1-lack of left ventricular hypertrophy
• 2-no previous MI (pre-existing scarring
tends to prevent myocardial tearing).
• 4-Pericarditis.
• A fibrinous or hemorrhagic pericarditis
usually develops within 2 to 3 days of a
transmural MI and typically spontaneously
resolves with time .
• 5-Infarct expansion.
• Because of the weakening of necrotic
muscle, there may be disproportionate
stretching, thinning, and dilation of the
infarct region (especially with anteroseptal
infarcts)
• 6-Mural thrombus.
• With any infarct, the combination of a local
loss of contractility (causing stasis) with
endocardial damage (causing a
thrombogenic surface) can foster mural
thrombosis and, potentially,
thromboembolism
• 7-Ventricular aneurysm.
• A late complication, aneurysms of the
ventricular wall most commonly result from
a large transmural anteroseptal infarct that
heals with the formation of thin scar tissue
• Complications of ventricular
aneurysms include :
• 1-mural thrombus
• 2-arrhythmias
• 3-heart failure
• 8-Papillary muscle dysfunction.
• dysfunction of a papillary muscle after MI
occurs due to:
• 1- as a result of rupture.
• 2- postinfarct mitral regurgitation results
from ischemic dysfunction of a papillary
muscle and underlying myocardium
• 3- papillary muscle fibrosis and shortening
• 4- ventricular dilation.
• 9-Progressive late heart failure
Long-term prognosis after MI
- depends on many factors:
- 1- left ventricular function
- 2- the severity of atherosclerotic narrowing
of vessels perfusing the remaining viable
myocardium.
Chronic Ischemic Heart Disease
• progressive heart failure as a consequence of
ischemic myocardial damage.
• In most instances there is a history of MI.
• Chronic IHD usually results from postinfarction
cardiac decompensation that follows exhaustion
of the hypertrophy of the viable myocardium.
• In other cases severe obstructive CAD may be
present without prior infarction, but with diffuse
myocardial dysfunction.
Clinical Features :
• Chronic IHD is characterized by the
development of severe, progressive heart
failure, sometimes punctuated by episodes
of angina or MI.
• Arrhythmias are common and, along with
CHF
Sudden Cardiac Death (SCD)
• Affecting some 300,000 to 400,000 individuals annually
in the United States,
• SCD is most commonly defined as unexpected death
from cardiac causes either without symptoms or
within 1 to 24 hours of symptom onset
• Coronary artery disease is the most common
underlying cause
• In many adults SCD is the first clinical manifestation of
IHD.
• With younger victims other nonatherosclerotic causes
are more common:
Other causes
•
•
•
•
•
•
•
1-Congenital coronary arterial abnormalities
2-Aortic valve stenosis
3-Mitral valve prolapse
4-Myocarditis or sarcoidosis
5-Dilated or hypertrophic cardiomyopathy
5-Pulmonary hypertension
6-Hereditary or acquired abnormalities of the cardiac
conduction system.
• 7-Isolated myocardial hypertrophy.
• 8-hypertensive
• 9-unknown cause.