* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download New Transfusions Transplantation Infections Rabies
Survey
Document related concepts
Cross-species transmission wikipedia , lookup
Ebola virus disease wikipedia , lookup
Orthohantavirus wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Oesophagostomum wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Hepatitis C wikipedia , lookup
West Nile fever wikipedia , lookup
Marburg virus disease wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Transcript
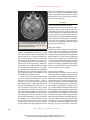
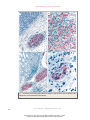
The new england journal of medicine original article Transmission of Rabies Virus from an Organ Donor to Four Transplant Recipients Arjun Srinivasan, M.D., Elizabeth C. Burton, M.D., Matthew J. Kuehnert, M.D., Charles Rupprecht, V.M.D., Ph.D., William L. Sutker, M.D., Thomas G. Ksiazek, D.V.M., Ph.D., Christopher D. Paddock, M.D., Jeannette Guarner, M.D., Wun-Ju Shieh, M.D., Ph.D., Cynthia Goldsmith, M.S., Cathleen A. Hanlon, V.M.D., Ph.D., James Zoretic, M.D., Bernard Fischbach, M.D., Michael Niezgoda, M.S., Waleed H. El-Feky, M.D., Lillian Orciari, M.S., Edmund Q. Sanchez, M.D., Anna Likos, M.D., M.P.H., Goran B. Klintmalm, M.D., Denise Cardo, M.D., James LeDuc, Ph.D., Mary E. Chamberland, M.D., M.P.H., Daniel B. Jernigan, M.D., M.P.H., and Sherif R. Zaki, M.D., Ph.D., for the Rabies in Transplant Recipients Investigation Team* abstract background In 2004, four recipients of kidneys, a liver, and an arterial segment from a common organ donor died of encephalitis of an unknown cause. methods We reviewed the medical records of the organ donor and the recipients. Blood, cerebrospinal fluid, and tissues from the recipients were tested with a variety of assays and pathological stains for numerous causes of encephalitis. Samples from the recipients were also inoculated into mice. results The organ donor had been healthy before having a subarachnoid hemorrhage that led to his death. Encephalitis developed in all four recipients within 30 days after transplantation and was accompanied by rapid neurologic deterioration characterized by agitated delirium, seizures, respiratory failure, and coma. They died an average of 13 days after the onset of neurologic symptoms. Mice inoculated with samples from the affected patients became ill seven to eight days later, and electron microscopy of central nervous system (CNS) tissue demonstrated rhabdovirus particles. Rabies-specific immunohistochemical and direct fluorescence antibody staining demonstrated rabies virus in multiple tissues from all recipients. Cytoplasmic inclusions consistent with Negri bodies were seen in CNS tissue from all recipients. Antibodies against rabies virus were present in three of the four recipients and the donor. The donor had told others of being bitten by a bat. From the Divisions of Healthcare Quality Promotion (A.S., D.C., D.B.J.) and Viral and Rickettsial Diseases (M.J.K., C.R., T.G.K., C.D.P., J.G., W.-J.S., C.G., C.A.H., M.N., L.O., J.L., M.E.C., S.R.Z.), National Center for Infectious Diseases, and the Epidemic Intelligence Service Branch, Division of Applied Public Health Training, Epidemiology Program Office (A.L.), Centers for Disease Control and Prevention, Atlanta; Baylor University Medical Center, Dallas (E.C.B., W.L.S., B.F., W.H.E., E.Q.S., G.B.K.); and the Texas Department of State Health Services, Austin (J.Z.). * Members of the Rabies in Transplant Recipients Investigation Team are listed in the Appendix. N Engl J Med 2005;352:1103-11. Copyright © 2005 Massachusetts Medical Society. conclusions This report documenting the transmission of rabies virus from an organ donor to multiple recipients underscores the challenges of preventing and detecting transmission of unusual pathogens through transplantation. n engl j med 352;11 www.nejm.org march 17, 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1103 The new england journal r abies is an acute encephalitis caused by viruses in the genus Lyssavirus, family Rhabdoviridae, that is nearly uniformly fatal in unvaccinated hosts. Although the virus is present in animal reservoirs, infection in humans is rare in the United States, with only two cases reported in 20031,2 and no more than six cases reported in any year in the past decade.3 The primary mode of transmission is through the bite of an infected animal, most commonly a bat in the United States.4 Although transmission of rabies virus from corneal transplants has previously been described,5 to our knowledge, no cases ascribed to organ or vascular-tissue transplants have been reported. In May 2004, physicians at a hospital in Texas diagnosed encephalitis in three recipients of a liver and two kidneys from a common organ donor. It was later discovered that encephalitis also developed in a fourth patient, who had received a vascular graft from the same donor during liver transplantation. All four patients became progressively obtunded, lapsed into coma, and died within 50 days after transplantation. The initial diagnostic evaluation revealed no cause of the encephalitis, and assistance was sought from the Centers for Disease Control and Prevention (CDC) and the Texas Department of State Health Services. We report the results of this investigation. case reports transplant recipients In May 2004, encephalitis was diagnosed in three recipients of a liver and two kidneys (Patients 2, 3, and 4 in Fig. 1) from a common organ donor. In all three patients, signs and symptoms of altered mental status and progressively worsening encephalitis developed within 30 days after transplantation. Major clinical events and immunosuppressive medications are summarized in Figure 1. All patients had rapid neurologic deterioration characterized by agitated delirium and seizures. Respiratory failure requiring intubation developed within 48 hours after the onset of neurologic symptoms. Examination of cerebrospinal fluid from the three patients showed pleocytosis, with an average of 18 cells per cubic millimeter (range, 7 to 35), and elevated protein levels (mean, 135 mg per deciliter; range, 17 to 331). Neurologic imaging in the week after the onset of symptoms showed no evidence of an acute cerebral process. Magnetic resonance imaging (MRI) performed later in the course of illness 1104 n engl j med 352;11 of medicine demonstrated diffuse signal abnormalities, most often in the temporal lobes, basal ganglia, brain stem, and hippocampi on T2-weighted and fluidattenuated inversion recovery images (Fig. 2). There was minimal enhancement after the administration of gadolinium. The patients died an average of 13 days after the onset of neurologic symptoms (range, 7 to 23). organ donor Four days before death, the organ donor was seen twice at an emergency department for nausea, vomiting, and difficulty swallowing. He was subsequently admitted to another hospital with altered mental status requiring intubation. Physical examination revealed a temperature of 38.1°C (100.5°F) and fluctuating blood pressures, including systolic measurements of more than 200 mm Hg. On admission, a urine toxicology screen was positive for cocaine and marijuana, and computed tomography of the brain demonstrated a subarachnoid hemorrhage. The hemorrhage progressed, and the neurologic symptoms, including seizures and coma, worsened. The patient was declared brain-dead within four days after presentation. Donor-eligibility screening and testing performed by an organ-procurement organization, including a review of premortem blood, urine, and sputum bacterial cultures, did not detect any signs or symptoms of infection precluding solid-organ donation. The patient’s kidneys, lungs, and liver were removed for transplantation; in addition, iliac arteries were harvested for potential use in vascular reconstruction during the liver transplantation. In part because of the positive toxicology result, nonorgan tissues (e.g., tendons) were not removed. During contact investigations conducted after the rabies diagnoses were made, friends of the donor indicated he had reported being bitten by a bat. methods clinical and epidemiologic review Medical records of the donor and infected transplant recipients were reviewed to characterize clinical courses and diagnostic evaluations. After the laboratory diagnosis of rabies infection in the three organ recipients, case finding was performed to search for other possible cases. Hospital autopsy records on patients with encephalitis were reviewed for pathological findings consistent with the presence of rabies. Also, charts of patients who had been www.nejm.org march 17 , 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. rabies transmission from an organ donor to four recipients Hospitalization Agitation, seizures, fever, sepsis Hemodynamic instability Delirium Home Organ or Tissue Transplanted Sepsis Patient 1 Iliac Artery X Incision and Tacrolimus, drainage mycophenolate of septic mofetil, abdominal prednisolone abscess Ventilation Death Hepatic-artery revision Fever Diffuse tremors, sleepiness Death Liver X Tacrolimus, Death mycophenolate mofetil, prednisolone X Ventilation Agitation, seizures Donor Mild Abdominal rejection flank pain Patient 3 Fever Death Kidney X Appendectomy Ventilation, hemodynamic instability Cyclosporine, sirolimus, prednisone Confusion, Fever Hemodynamic agitation, instability myoclonus Patient 4 Kidney X 19 ne ne Ju 16 Ju 13 ne Ju 10 ne Ju ne Ju Ju ne 4 1 ne ne Ju 29 Ju 26 ay ay M 23 M M ay 20 17 ay M ay M ay 14 11 7 Transplant nephrectomy, immunosuppression stopped Ventilation M 8 ay ay M 5 M 2 ay M M ay ril 29 Tacrolimus, mycophenolate mofetil, prednisone Ap Death 22 Patient 2 Hemodynamic instability Figure 1. The Clinical Course of Four Recipients of Rabies-Infected Tissue or Organs. matoxylin and eosin and various immunohistochemical stains according to a method described previously.6 For immunohistochemical assays, 3-µm tissue sections were deparaffinized, rehydrated, and digested in proteinase K. Tissue sections were incubated for 60 minutes at room temperature with a hyperimmune rabbit antiserum or mouse laboratory methods ascitic fluid with reactivity to rabies virus. After Formalin-fixed, paraffin-embedded tissue speci- sequential application of the appropriate biotinymens, obtained at autopsy, were stained with he- lated linked antibody, avidin–alkaline phosphatase on the same floor as a patient with rabies and who had also had a lumbar puncture or neurology consultation for altered mental status were examined for documented clinical findings consistent with the presence of rabies. Procedures for organ recovery and handling were also reviewed. n engl j med 352;11 www.nejm.org march 17, 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1105 The new england journal of medicine fluorescence antibody assay according to a previously described method.9 Immunohistochemical studies were performed as described above, and formalin-fixed tissues were embedded for examination by electron microscopy. results review of transplantation records Figure 2. Axial Fluid-Attenuated Inversion Recovery MRI Scan Showing Profound Signal Abnormalities within the Bilateral Frontal and Temporal Lobes, Hippocampi, Basal Ganglia, and Medulla in Patient 2. complex, and naphthol fast-red substrate, sections were counterstained in Meyer’s hematoxylin and mounted with the use of aqueous mounting medium. Serologic analyses, detection of viral antigen in tissue by means of fluorescence microscopy, and identification of rabies virus variants were performed as described previously.7,8 Controls included serum specimens from noninfected animals, tissues from humans with nonrabies encephalitides, and rabies-infected human tissues. Immunohistochemical assays for various other viral, rickettsial, and protozoan agents of encephalitis were also performed on tissues from recipients. Vero E6 cells were inoculated with CSF and 10 percent tissue suspensions from three of the four rabies-infected recipients (Patients 2, 3, and 4). Suckling mice were inoculated intracranially and intraperitoneally with cerebrospinal fluid and 10 percent clarified homogenates of brain tissue, spinal cord, and kidney suspensions. Tissue cultures and suckling mice were observed daily for cytopathic effects and signs of illness, respectively. Tissues obtained from suckling mice that developed neurologic signs or died were fixed in 10 percent neutral buffered formalin or 2.5 percent buffered gluteraldehyde or were frozen for further evaluation. At 14 days, the Vero E6 cells were suspended in saline, fixed on glass slides, and tested for the presence of rabies virus antigen by means of a direct 1106 n engl j med 352;11 All organs obtained from the donor were transplanted; the lung recipient died of intraoperative complications. Iliac arteries from the donor were not used during the liver transplantation in Patient 2 and were placed in a sterile container and stored for potential use in subsequent transplantation procedures. One day after the organs were transplanted, the iliac-artery segment was retrieved and used to construct a vascular graft for another liver-transplant procedure (in Patient 1). rabies case finding In addition to the three initial cases noted by physicians, autopsy review identified a fourth patient (Patient 1 in Fig. 1) in whom progressive, fatal encephalitis had developed after liver transplantation. This patient had received the vascular segment from the rabies-infected donor. A review of the medical records of patients who had been on the same floor as a patient with rabies and who had had a lumbar puncture or neurology consultation for altered mental status revealed no further cases of encephalitis consistent with the presence of rabies. pathological findings Histopathological evaluation of tissues from all four rabies-infected transplant recipients demonstrated diffuse, predominantly lymphohistiocytic, infiltrates and microglial nodules involving the cerebrum, brain stem, cerebellum, and spinal cord. Cytoplasmic inclusions consistent with Negri bodies were identified throughout the central nervous system (CNS), particularly in the Purkinje cells of the cerebellum and in neurons of the frontal cortex, thalamus, hippocampus, midbrain, and pons (Fig. 3A). Lymphohistiocytic infiltrates involving the peripheral nerves, heart, and kidneys were also noted in some patients. Electron microscopy of the midbrain of Patient 4 demonstrated abundant rhabdovirus particles (Fig. 3B). Intracytoplasmic rabies virus antigens were detected on immunohistochemical staining in neurons from multiple areas of the CNS (Fig. 3C); in peripheral nerves of the trans- www.nejm.org march 17 , 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. rabies transmission from an organ donor to four recipients planted kidneys, liver, and arterial graft (Fig. 4); and in renal tubular epithelium, smooth muscle, histiocytes, and vascular endothelium. No tissues were positive for enteroviruses, human herpesviruses 1 and 2, West Nile and other flaviviruses, eastern equine encephalomyelitis virus, lymphocytic choriomeningitis virus, Cache Valley virus, henipaviruses, measles virus, spotted fever and typhus group rickettsiae, Toxoplasma gondii, or Trypanosoma cruzi on immunohistochemical analysis. Direct fluorescence antibody staining also demonstrated rabies virus antigens in CNS tissues from all recipients. A C serologic analyses and viral identification Antibodies (IgM and IgG) reactive to rabies virus were present in the donor’s serum at the time of death. Antibodies were also present in three of the four recipients in samples obtained on postoperative days 35 and 36; both IgM and IgG antibodies were present in one kidney recipient (Patient 3) and the recipient of the donor’s liver (Patient 2), whereas only IgG antibodies were present in the patient who received the arterial segment (Patient 1). Antigenic typing revealed a previously characterized rabies virus variant associated with bats. B cell culture and mouse inoculations All suckling mice had neurologic abnormalities or had died seven to eight days after inoculation. Thinsection electron microscopy of CNS tissue demonstrated rhabdovirus particles, and IHC testing detected rabies virus antigens in mouse CNS tissues. Cultures of Vero E6 cells inoculated with brain, spinal cord, and kidney from a kidney recipient demonstrated rabies virus antigen on staining with DFA. discussion This report describes the transmission of rabies virus through the transplantation of solid organs and vascular material. Four patients who received transplants — three organs and one vascular segment — from a donor with unrecognized rabies infection subsequently died of rabies. The transmission of rabies from corneal transplants has been described previously.5 Rabies is seldom included in the differential diagnosis of encephalitis in the absence of a documented exposure or suggestive history.8,10 The symptoms in the cases reported here, including fever, changes in mental status, and autonomic n engl j med 352;11 Figure 3. Histopathological Findings in Patient 4. Panel A shows multiple intracytoplasmic viral inclusions (Negri bodies, arrows) in neurons in the CNS of Patient 4, who received a kidney from the rabiesinfected donor. In Panel B, an electron micrograph shows typical inclusions in the midbrain. Virions are sectioned longitudinally (arrowhead) and transversely (arrows). The virions averaged 65 nm in diameter. Panel C shows immunohistochemical staining (red) of rabies virus antigens in the CNS. instability, were, in retrospect, consistent with a diagnosis of rabies. However, the diagnosis was complicated by the absence of a history of exposure at presentation and by the number of other potential causes of illness in these immunosuppressed patients. A history of a bat bite in the donor was discovered during contact interviews only after rabies had been diagnosed, and the investigation initiated. The diagnosis in the donor was further complicated by the presence of a subarachnoid hemor- www.nejm.org march 17, 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1107 The new england journal A B C D of medicine Figure 4. Immunohistochemical Staining (Red) of Rabies Virus Antigens in Peripheral Nerves of the Liver (Panels A and B), Kidney (Panel C), and Arterial-Graft Transplants (Panel D). 1108 n engl j med 352;11 www.nejm.org march 17 , 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. rabies transmission from an organ donor to four recipients rhage in the setting of hypertension and a positive toxicology screen for cocaine. It is not known whether rabies infection was the cause of the subarachnoid hemorrhage, since this finding has not been noted in previous reports.11-13 Signs of rabies developed in all four transplant recipients within 30 days after infection. According to previous reports, symptoms developed within 30 days after an animal bite in only 25 percent of patients.10 It is unknown whether the shorter incubation period in these patients was due to the immunosuppression, the route of transmission, or both. The effect of immunosuppression on rabies infection is currently not well understood. In reports of rabies transmission from corneal transplants in patients who were not immunosuppressed and did not receive postexposure prophylaxis, symptoms developed an average of 26 days after transplantation,14-17 suggesting that implantation of material from infected donors may lead to a shorter incubation period. Three of our patients presented with commonly described symptoms of tremors and changes in mental status, whereas the fourth presented with abdominal and flank pain, which may have been neuropathic, and changes in mental status occurred about 48 hours later. The rapidly progressive encephalitis, with death occurring an average of 13 days after the onset of symptoms, is consistent with the course in other reports.4 There is only one reported case of recovery from clinical rabies by a patient who had not received preexposure or postexposure prophylaxis against rabies.18 However, administration of postexposure prophylaxis with rabies immune globulin and vaccine is highly effective in preventing infection after exposure. In a previous report, administration of postexposure prophylaxis probably prevented infection in a patient who had received a cornea from a donor with rabies.19 This report and another, describing the transmission of West Nile virus through solid-organ transplantation,20 underscore the potential for transmission of unexpected infectious diseases through organ transplantation. Recognition and prevention of transplant-transmitted infections may be improved in various ways, including enhanced donor screening and testing, the development of standardized procedures related to storage and use of donor vascular segments, as well as methods to track their use or nonuse, and enhanced means of detection and diagnosis of illnesses in recipients. To minimize the risk of transmitting infections n engl j med 352;11 during organ transplantation, the Organ Procurement and Transplantation Network (OPTN) has established standards that require organ-procurement organizations to assess the risks of infectious diseases through screening questions and blood testing for selected bloodborne viral pathogens and syphilis.21 Questions about potential exposure to rabies are generally not included, and laboratory testing for rabies infection is not performed. Organs can be procured from donors who are febrile, provided that the medical director of the organ-procurement organization and the transplantation physicians agree that the cause of the fever does not pose an unacceptable risk to the recipient. Given the growing importance of emerging and reemerging infectious diseases, the ability of general improvements in the donor-screening process, rather than disease-specific measures, to increase organ safety should be evaluated. A proposed revision of OPTN policies would expand the list of potentially transmittable diseases and conditions that clinicians should consider in determining a donor’s eligibility.22 The revision emphasizes that when any of these conditions is known or suspected in a donor, this information should be conveyed immediately to the organ-procurement organization as well as to all transplantation centers that received organs from the donor. The successful use of donor arterial conduits has been reported in liver transplantation23-26 and in the management of vascular complications in recipients of both hepatic transplants27,28 and renal transplants.29 As with organs, these vessel segments have the potential to transmit infection. A careful accounting of and an ability to track donated material, such as vessel conduits, are essential in efforts to link unexplained illnesses or deaths to a common organ donor and will increase the probability of quickly identifying all recipients who may be at risk from donor infections. Proposed revisions of the policies of both OPTN22 and the Joint Commission on Accreditation of Healthcare Organizations30 may help address the storage of vessel conduits and documentation of their use or nonuse. Our investigation underscores the challenge in detecting and diagnosing infections that occur in recipients of organs or tissues from a common donor. The potential for disease transmission from a donor as a cause of illness or death may not be considered in the evaluation of an individual recipient. In this investigation, and in the previous report of the transmission of West Nile virus through www.nejm.org march 17, 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1109 The new england journal transplantation, the ability to connect illnesses to a common organ donor was facilitated by the fact that multiple recipients were hospitalized at the same facility. Improved national detection of unexpected or serious outcomes among transplant recipients may facilitate the discovery of transplant-related transmission of emerging and unusual pathogens by allowing connections to common donors to be made. The ability to make retrospective diagnoses of infections in organ donors when unexplained deaths or illnesses occur in recipients is hampered by the limited availability of donor samples, particularly tissue; currently, only serum samples from organ donors are retained for any length of time. Investigations into possible transplantation-associated infections would be facilitated by the availability of selected, archived tissue samples from the donor and by autopsy reports and materials. An improved diagnostic ability may have important implications for other patients who received material from the donors and for contacts of the patients and donors. As organ and tissue transplantation becomes of medicine more common, the potential risks of disease transmission may increase. Cases of transplantationassociated infections provide important opportunities to review practices in an attempt to enhance the safety of transplantation without affecting the organ supply. The Department of Health and Human Services, including the CDC, is working with other partners in the organ- and tissue-transplantation community to review donor-screening practices, the use of retained vascular segments, and surveillance of recipients for illness. Clinicians who care for organ-transplant recipients should continue to be aware of the potential for disease transmission through transplantation and the challenges in recognizing atypical presentations of infections in this immunosuppressed population. Clinicians should report unexpected outcomes or unexplained illnesses in transplant recipients to their local organand tissue-procurement organization. We are indebted to the state health departments in Oklahoma and Alabama, to the Southwest Transplant Alliance, and to the staff of the Baylor University Medical Center for their assistance with this investigation. appendix The other members of the Rabies in Transplant Recipients Investigation Team are as follows: J.A. Comer, J. Dillaha, B. Flow, D. Johnson, J. McQuiston, M. J. Opatowsky, N. Pascoe, D. Perrotta, P. Rollin, D. Swerdlow, B. Tierney, J. D. Walker, F. Wilson, and P. Yager. references 1. First human death associated with rac- coon rabies — Virginia, 2003. MMWR Morb Mortal Wkly Rep 2003;52:1102-3. 2. Human death associated with bat rabies — California, 2003. MMWR Morb Mortal Wkly Rep 2004;53:33-5. 3. Cases of rabies in human beings in the United States, by circumstances of exposure and rabies virus variant, 1990-2001. (Accessed February 23, 2005, at http://www. cdc.gov/ncidod/dvrd/rabies/professional/ publications/Surveillance/Surveillance01/ Table2-01.htm.) 4. Bleck TP, Rupprecht CE. Rabies virus. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. Philadelphia: Churchill Livingstone, 2000: 1811-20. 5. Doe J. Human rabies prevention — United States, 1999: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999; 48(RR-1):1-21. [Errata, MMWR Recomm Rep 1999;48:16, 2000;49:737.] 6. Zaki SR, Greer PW, Coffield LM, et al. Hantavirus pulmonary syndrome: pathogen- 1110 esis of an emerging infectious disease. Am J Pathol 1995;146:552-79. 7. Warner CK, Zaki SR, Shieh WJ, et al. Laboratory investigation of human deaths from vampire bat rabies in Peru. Am J Trop Med Hyg 1999;60:502-7. 8. Noah DL, Drenzek CL, Smith JS, et al. Epidemiology of human rabies in the United States, 1980 to 1996. Ann Intern Med 1998; 128:922-30. 9. Protocol for postmortem diagnosis of rabies in animals by direct fluorescent antibody testing: a minimum standard for rabies diagnosis in the United States. (Accessed February 23, 2005, at http://www.cdc.gov/ncidod/ dvrd/rabies/professional/publications/DFA_ diagnosis/DFA_protocol-b.htm.) 10. Anderson LJ, Nicholson KG, Tauxe RV, Winkler WG. Human rabies in the United States, 1960 to 1979: epidemiology, diagnosis, and prevention. Ann Intern Med 1984; 100:728-35. 11. Esiri MM, Kennedy PGE. Virus diseases. In: Adams JH, Duchen LW, eds. Greenfield’s neuropathology. 5th ed. New York: Oxford University Press, 1992:335-99. n engl j med 352;11 www.nejm.org 12. Awasthi M, Parmar H, Patankar T, Castillo M. Imaging findings in rabies encephalitis. AJNR Am J Neuroradiol 2001; 22:677-80. 13. Mani J, Reddy BC, Borgohain R, Sitajayalakshmi S, Sundaram C, Mohandas S. Magnetic resonance imaging in rabies. Postgrad Med J 2003;79:352-4. 14. Human-to-human transmission of rabies via corneal transplant — Thailand. MMWR Morb Mortal Wkly Rep 1981;30:473-4. 15. Houff SA, Burton RC, Wilson RW, et al. Human-to-human transmission of rabies virus by corneal transplant. N Engl J Med 1979;300:603-4. 16. Gode GR, Bhide NK. Two rabies deaths after corneal grafts from one donor. Lancet 1988;2:791. 17. Human-to-human transmission of rabies via a corneal transplant — France. MMWR Morb Mortal Wkly Rep 1980;29:25-6. 18. Recovery of a patient from clinical rabies, Wisconsin 2004. MMWR Morb Mortal Wkly Rep 2004;53:1171-3. 19. Sureau P, Portnoi D, Rollin P, Lapresle C, Lapresle C, Chaouni-Berbich A. Prevention march 17 , 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. rabies transmission from an organ donor to four recipients of inter-human rabies transmission after corneal graft. C R Seances Acad Sci III 1981; 293:689-92. (In French.) 20. Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med 2003;348:2196-203. 21. Organ Procurement and Transplantation Network. Minimum procurement standards for an organ procurement organization. (Accessed February 23, 2005, at http:// www.optn.org/policiesandbylaws.) 22. Organ Procurement and Transplantation Network Policy Proposal. (Accessed February 23, 2005, at http://www.unos.org/ policiesAndBylaws/publicComment/pdfs/ pubcommentProp_15.pdf.) 23. Zamboni F, Franchello A, Ricchiuti A, Fop F, Rizzetto M, Salizzoni M. Use of arte- rial conduits as an alternative technique in arterial revascularization during orthotopic liver transplantation. Dig Liver Dis 2002;34: 122-6. 24. Muralidharan V, Imber C, Leelaudomlipi S, et al. Arterial conduits for hepatic artery revascularization in adult liver transplantation. Transpl Int 2004;17:163-8. 25. Goldstein RM, Secrest CL, Klintmalm GB, Husberg BS. Problematic vascular reconstruction in liver transplantation. I. Arterial. Surgery 1990;107:540-3. 26. Muiesan P, Rela M, Nodari F, et al. Use of infrarenal conduits for arterial revascularization in orthotopic liver transplantation. Liver Tranpl Surg 1998;4:232-5. 27. Sellers MT, Haustein SV, McGuire BM, et al. Use of preserved vascular homografts in liver transplantation: hepatic artery aneu- rysms and other complications. Am J Transplant 2002;2:471-5. 28. Pinna AD, Smith CV, Furukawa H, Starzl TE, Fung JJ. Urgent revascularization of liver allografts after early hepatic artery thrombosis. Transplantation 1996;15:1584-7. 29. Shames BD, Odorico JS, D’Alessandro AM, Pirsch JD, Sollinger HW. Surgical repair of transplant renal artery stenosis with preserved cadaveric iliac artery grafts. Ann Surg 2003;237:116-22. 30. Joint Commission on Accreditation of Healthcare Organizations. Proposed Tissue Storage and Issuance Standards. (Accessed February 23, 2005, at http://www.jcaho.org/ accredited+organizations/hospitals/ standards/field+reviews/tissue_fr_stds.htm.) Copyright © 2005 Massachusetts Medical Society. images in clinical medicine The Journal welcomes consideration of new submissions for Images in Clinical Medicine. Instructions for authors and procedures for submissions can be found on the Journal ’s Web site at www.nejm.org. At the discretion of the editor, images that are accepted for publication may appear in the print version of the Journal, the electronic version, or both. n engl j med 352;11 www.nejm.org march 17, 2005 Downloaded from www.nejm.org at TX DEPT OF HEALTH on March 17, 2005 . Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1111