* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHM121 Exam I Review
Survey
Document related concepts
Rigid rotor wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Calcium looping wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Rate equation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic nucleus wikipedia , lookup
Stoichiometry wikipedia , lookup
Metalloprotein wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Transcript
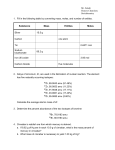
California State Polytechnic University, Pomona General Chemistry, CHM 121, Dr. Laurie S. Starkey Exam I Review, Chapters 1–3 General Topics: significant figures, scientific notation, SI base unit & prefix, density, conversion factors, periodic table (periods/groups), chemical formula, balancing equations, nomenclature, molecular/formula weight, percent composition (mass percent), stoichiometry. Be able to define the following terms: states of matter, element, compound, ionic vs. molecular compounds, atom, atomic symbol, nucleus, electron, proton, atomic number, neutron, mass number, nuclide, isotopes, atomic weight, metal, nonmetal, metalloid, ion, cation, anion, chemical reaction, reactant, product, mole, Avogadro's number, empirical formula, limiting reactant/reagent, theoretical yield, percent yield. 1. Complete the following conversion factors: 1 m = ______ cm 1 mL = _______ L 1 L = _______ mL 1 g = _______ kg 1 kg = _______ g 1. What is the mass number of carbon-13? What is the nuclide symbol for carbon-13? 2. How many neutrons does iodine-131 (131I) have? 3. How many electrons are in each of the following atoms? Mn __________ Br–__________ potassium ion __________ Fe2+__________ copper (III) __________ 4. What is the charge of the nucleus of a calcium atom? of a calcium ion? 5. What is the weight of a 165 pound person in grams? (1 lb = 0.4536 kg) 6. A procedure calls for 6.5 g thionyl chloride (SOCl2). SOCl2 is a liquid so you want to measure it using a syringe. Its density is 1.631, according to an Aldrich catalog. How many mL are required? 7. How many chlorine atoms are in 6.5 g thionyl chloride (SOCl2)? 8. What are the formulas for the following anions: nitrite? nitride? nitrate? 9. Which name is correct for Ca3(PO4)2? Explain what is wrong with each incorrect name. a. calcium (II) phosphate b. calcium (III) phosphate c. tricalcium diphosphate d. calcium (II) phosphite e. calcium phosphite f. calcium phosphate 10. The combustion of a 1.50 g sample yields 1.17 g H2O. What is the mass % of H in the sample? 11. What is the mass percent of carbon in the fuel octane (C8H18)? 12. Balance the following equation. When 5.00 g of Sn were combined with 5.00 g HNO3, an 82% yield of SnO2 was obtained. How much SnO2 (in grams) was produced in that reaction? _____Sn + _____HNO3 13. Fill in the missing blanks. → _____SnO2 + _____NO2 + _____H2O nuclide symbol 44 20 # of protons # of electrons # of neutrons Ca2+ 14. Provide the molecular formula and empirical formula for each of the following. H O H H C C H H O H H H H H C C C H H H H H H H C C H H C C H H H mol. form. _________ mol. form. _________ mol. form. _________ emp. form._________ emp. form._________ emp. form._________ 15. Name or give formulas for each of the following compounds. Formula Name Name Formula Zn3(PO4)2 ______________________________ potassium sulfate ____________ NH4OH ______________________________ sulfuric acid ____________ calcium carbonate ____________ 16. A compound contains 1.36 mol Li, 2.72 mol B and 4.76 mol O. What is its empirical formula? 17. Balance the following equation for the combustion of propane (BBQ fuel). _____C3H6 + _____O2 → _____H2O + _____CO2 a. If 0.50 moles of each starting material were combined, what is the theoretical yield of carbon dioxide (in grams)? b. How many grams of oxygen are required to burn 20 pounds of propane (one tankful). (1 kg = 2.2 lbs.)