* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Enthalpy change - Don`t Trust Atoms
Electron configuration wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Ionic liquid wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Heat transfer physics wikipedia , lookup
Electrochemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Marcus theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical bond wikipedia , lookup
Transition state theory wikipedia , lookup
Ionic compound wikipedia , lookup
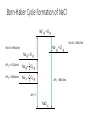
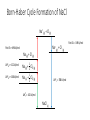
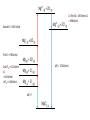
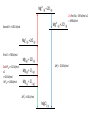
12 Thermodynamics 12.1 Types of Enthalpy Change 12.2 Born-Haber Cycles 12.3 Enthalpy Changes – Enthalpy of Solution 12.4 Mean Bond Enthalpy 12.5 Entropy 12.1 Enthalpy Change – Ionic Compounds Learning Objectives: 1. Describe what is meant by the term enthalpy change. 2. Describe the different types of enthalpy changes (formation, atomisation, ionisation energy, electron affinity, lattice formation, hydration, solution, bond enthalpy). 3. Calculate the enthalpy changes on forming ionic compounds. Enthalpy Review • Enthalpy change is the heat change at constant pressure. • Standard conditions: 100kPa, 298 K (starting temperature) • Remember that heat and temperature are not the same. • Heat is a type of energy and is measured in joules and heat changes lead to temperature changes, which is measure in Kelvins. Types of Enthalpy Changes • Enthalpy of Formation • Enthalpy of Atomisation • First Ionisation Energy/Second Ionisation Energy • First Electron Affinity/Second Electron Affinity • Lattice Enthalpy of Formation • Enthalpy of Lattice Dissociation • Enthalpy of Hydration Write down the symbol • Enthalpy of Solution and the definition • Mean Bond Enthalpy Standard Enthalpy of Formation change ∆HꝊf standard conditions formation • Enthalpy change when • one mole of a compound is formed • from its constituent elements • under standard conditions • all reactants and products in their standard states. Standard Enthalpy of Atomisation ∆HꝊat • Enthalpy change when • one mole of gaseous atoms • is formed • from the element • In it’s standard state • under standard conditions First Ionisation Energy First IE • Enthalpy change when • one mole of gaseous atoms • is converted into • one mole of gaseous +1 ions • under standard conditions Second Ionisation Energy Second IE • Enthalpy change when • one mole of gaseous +1 ions • is converted into • one mole of gaseous +2 ions • under standard conditions First Electron Affinity First ∆HꝊea • Enthalpy change when • one mole of gaseous atoms • is converted into • one mole of gaseous -1 ions • under standard conditions Second Electron Affinity Second ∆HꝊea • Enthalpy change when • one mole of gaseous -1 ions • is converted into • one mole of gaseous -2 ions • under standard conditions Lattice Formation Enthalpy ∆HꝊL • Enthalpy change when • one mole of solid ionic compound • is formed • from it’s gaseous ions • under standard conditions • (always negative, energy released) Enthalpy of Lattice Dissociation -∆HꝊL • Enthalpy change when • one mole of solid ionic compound • dissociates into • it’s gaseous ions • under standard conditions • (always positive, energy is absorbed) Standard Enthalpy of Hydration ∆HꝊhyd • Enthalpy change when • one mole of gaseous atoms • is surrounded by water molecules • under standard conditions Standard Enthalpy of Solution ∆HꝊsol • Enthalpy change when • one mole of solute • completely dissolves • in sufficient solvent to form a solution in which the molecules are ions do not interact • under standard conditions Mean Bond Enthalpy ∆HꝊdiss • Enthalpy change when • one mole of gaseous molecules • breaks a covalent bond • forming two free radicals • averaged over a range of compounds • at standard conditions For each type… a) Write an equation to represent the chemical reaction being described b) Tell me if the process is likely to be positive or negative c) Explain why. Standard Enthalpy of Formation H2 (g) + 1 O2 (g) 2 ∆HꝊf H2O (l) Usually going to be negative. Molecules usually form because the molecule is more stable (lower in energy) than the constituent elements. Remember: Bond making releases energy. Standard Enthalpy of Atomisation 1 Br2(l) 2 ∆HꝊat Br (g) Usually positive. Molecules form because that is a more stable form, so gaseous atoms are less stable (higher in energy). Remember: Bond breaking require energy. First Ionisation Energy First IE Na (g) Na+(g) + e- • Positive • Removing an electron takes energy Second Ionisation Energy Second IE Na+ (g) Na2+(g) + e- • Very positive • Removing electron from positive ion require a lot of energy. First Electron Affinity First ∆HꝊea O (g) + e- O- (g) • Usually Negative • Energy is gained when electrons are added. Second Electron Affinity Second ∆HꝊea O- (g) + e- O2- (g) • Usually Positive • Because of repulsion, adding the second electron requires more energy than is gained. Lattice Formation Enthalpy ∆HꝊL Na+ (g) + Cl- (g) NaCl (s) • Always negative • Bond making releases energy, more stable in lattice form. Enthalpy of Lattice Dissociation -∆HꝊL NaCl (s) Na+ (g) + Cl- (g) • Always positive • This is opposite of lattice formation, breaking bonds requires energy. Standard Enthalpy of Hydration ∆HꝊhyd Na+ (g) + aq Na+ (aq) Cl- (g) + aq Cl- (aq) • Usually negative • Water molecules stabilise the charges of the ions. Standard Enthalpy of Solution ∆HꝊsol NaCl (s) + aq Na+ (aq) + Cl- (aq) • Usually slightly positive • Breaking the bonds of the lattice requires energy, however, the water molecules stabilise the ions so overall only small amount of energy absorbed. Mean Bond Enthalpy ∆HꝊdiss CH4 (g) C (g) + 4H (g) • Always positive • Bond breaking requires energy. 12.2 Born-Haber Cycles Learning Objectives: 1. Describe Hess’ Law. 2. Use Born-Haber Cycles to calculate enthalpy changes Hess’s Law of Thermodynamics • The enthalpy change for a reaction is the same, no matter what route is taken. • For example: CH4 (g) + O2 (g) CO2 (g) + H2O (g) C (s) + H2 (g) + O2 (g) Born-Haber Cycles • Born-Haber Cycles are just another method to solve for the unknown enthalpy change of a chemical reaction by using enthalpy changes that we DO know. • It uses a diagram to represent the enthalpy changes on a vertical scale. Increases in energy are UP ( ) arrows, decreases in energy are DOWN ( )arrows. • Molly started out with £0. Then she received £100 for her birthday. She went out to dinner, this costed £30. Then she bought some new shoes. At the end of the day to had spent all of her birthday money. How much did her new shoes cost? With Birthday Money ∆Mdin = -£30 After Dinner ∆Mbd = +£100 ∆Mshu = ? = -£70 Broke Formation of an Ionic Compound • Electrons are transferred to atoms to form ions. • Ions then attract and are arranged into an ionic lattice. • This is how ionic lattices are formed. Enthalpy Change in Formation of Ionic Compounds 1 2 Na (s) + Cl2 (g) NaCl (s) ∆HꝊf = ? What are the steps for this complete reaction to occur? 1) Atomisation of Na 2) Atomisation of Chlorine 3) Ionisation of Na 4) Electron affinity of Cl 5) Formation of lattice Enthalpy Change in Formation of Ionic Compounds 1 2 Na (s) + Cl2 (g) NaCl (s) ∆HꝊf = ? What are the steps for this complete reaction to occur? 1) Atomisation of Na Na (s) Na (g) 2) 3) 4) 5) Atomisation of Chlorine Ionisation of Na Electron affinity of Cl Formation of lattice 1 2 Cl2 (g) Cl (g) Na (g) Na+ (g) Cl (g) Cl- (g) Na+ (g) + Cl- (g) NaCl (s) Enthalpy Change in Formation of Ionic Compounds 1 2 Na (s) + Cl2 (g) NaCl (s) ∆HꝊf = ? What are the steps for this complete reaction to occur? 1) Atomisation of Na Na (s) Na (g) ∆HꝊat = +108 kJ/mol 2) 3) 4) 5) Atomisation of Chlorine Ionisation of Na Electron affinity of Cl Formation of lattice 1 2 Cl2 (g) Cl (g) Na (g) Na+ (g) Cl (g) Cl- (g) Na+ (g) + Cl- (g) NaCl (s) ∆HꝊat = +122 kJ/mol first IE = +496 kJ/mol first EA = -349 kJ/mol ∆HꝊL = -788 kJ/mol Born-Haber Cycle Formation of NaCl Na+ (g) + Cl (g) First EA = -349 kJ/mol Na+ (g) + Cl- (g) First IE = +496 kJ/mol Na (g) + Cl (g) 1 2 1 (s) + 2 Cl2 (g) ∆HꝊat = +122 kJ/mol Na (g) + Cl2 (g) ∆HꝊat = +108 kJ/mol Na ∆HꝊL = -788 kJ/mol ∆HꝊf = ? NaCl (s) Born-Haber Cycle Formation of NaCl Na+ (g) + Cl (g) First EA = -349 kJ/mol Na+ (g) + Cl- (g) First IE = +496 kJ/mol Na (g) + Cl (g) 1 2 1 (s) + 2 Cl2 (g) ∆HꝊat = +122 kJ/mol Na (g) + Cl2 (g) ∆HꝊat = +108 kJ/mol Na ∆HꝊL = -788 kJ/mol ∆HꝊf = -411 kJ/mol NaCl (s) Example: Lattice Formation Enthalpy of MgCl2 • Write out the overall equation for the formation of magnesium chloride. • Write equations for all of the steps in the formation of magnesium chloride. • HINT: there are six steps Draw a Born-Haber Diagram for MgCl2 • HINT: there is a “trick” step, can you catch it? Remember your definitions • ∆HꝊat Mg = +148 kJ/mol • ∆HꝊat Cl = +122 kJ/mol • First IE Mg= +738 kJ/mol • Second IE Mg = +1451 kJ/mol • First EA Cl = -349 kJ/mol • ∆HꝊf MgCl2 = -641 kJ/mol Use your Born-Haber Diagram to Calculate the Lattice Formation Enthalpy Mg2+ (g) + 2Cl (g) Mg2+ (g) + 2Cl- (g) Second IE = +1451 kJ/mol Mg+ (g) + 2Cl (g) First IE = +738 kJ/mol Mg (g) + 2Cl (g) 2 x ∆HꝊat = +122 kJ/mol x2 = +244 kJ/mol ∆HꝊat = +148 kJ/mol ∆HꝊL = -2524 kJ/mol Mg (g) + Cl2 (g) Mg (s) + Cl2 (g) ∆HꝊf = ? MgCl2 (s) 2 x First EA = -349 kJ/mol x 2 = -698 kJ/mol Mg2+ (g) + 2Cl (g) Mg2+ (g) + 2Cl- (g) Second IE = +1451 kJ/mol Mg+ (g) + 2Cl (g) First IE = +738 kJ/mol Mg (g) + 2Cl (g) 2 x ∆HꝊat = +122 kJ/mol x2 = +244 kJ/mol ∆HꝊat = +148 kJ/mol ∆HꝊL = -2524 kJ/mol Mg (g) + Cl2 (g) Mg (s) + Cl2 (g) ∆HꝊf = -641 kJ/mol MgCl2 (s) 2 x First EA = -349 kJ/mol x 2 = -698 kJ/mol 12.3 More Enthalpy Changes Learning Objectives: 1. Calculate enthalpy change of solution. 2. Describe how lattice enthalpy calculations support models for ionic bonding. 3. Explain how ions can become polarised. Enthalpy of Solution • Ionic solids can dissolve in polar solvents. • This is called hydration if the solvent is water. • Hydration is when the water molecules surround ions. • What are the steps for process of forming a solution? 1. Breaking the ionic lattice (enthalpy of lattice dissociation). 2. Hydrating the positive ions (enthalpy of hydration). 3. Hydrating the negative ions (enthalpy of hydration). Example: NaCl Ionic Bonding Models • For most ionic compounds the theoretical values calculated from Born-Haber cycles agrees with experimental values. • This proves that the model for ionic bonding (lattice) is correct. • However, some ionic compounds have theoretical and experimental values that DO NOT agree. • Another model needed to be found to explain these discrepancies. Polarisation • ZnSe • experimental lattice formation enthalpy = -3611 kJ/mol • theoretical lattice formation enthalpy = -3305 kJ/mol WHY? • Zn2+ is very small and has a high + charge • Se2- is very large and has a high – charge • Zn2+ moves closely to electron density of Se2- and attracts the e• Since Se2- is large, the e- are far from the nucleus and easily pulled away • This distorts the electron cloud surrounding Se2- Polarisation • The distortion causes their to be some electron density shared between the two ions (slightly covalent nature). • The Se2- ion is said to be polarised. • This causes the enthalpy change to be greater than expected. When does polarisation happen? •Cation = small size, high charge •Anion = large size, high charge 12.4 Mean Bond Enthalpy Learning Objectives: 1. Explain the term mean bond enthalpy. 2. Calculate enthalpy changes using mean bond enthalpy. 3. Explain why this method is not as accurate. Mean Bond Enthalpy • The average bond enthalpy term is the average amount of energy needed to break a specific covalent bond, measured over a wide variety of different molecules. • A measure of strength of a covalent bond. • In comparison, lattice enthalpy is a measure of the strength of an ionic bond. Predicting reactions • Mean bond enthalpies can be used to predict how molecules may react. • We can predict which bonds may be more likely to break. Which bond is most likely to break? C-H C-C C-Br 413 kJ/mol 437 kJ/mol 290 kJ/mol Predicting reactions • Mean bond enthalpies can also be used to compare reactivities of different molecules. • Which haloalkane is more reactive? C-F C-Cl C-Br C-I 467 kJ/mol 346 kJ/mol 290 kJ/mol 228 kJ/mol Calculating Approximate Enthalpy Changes • Hess’s Law can be applied. • One possible route to products would be to break all bonds in the reactants and then form all of the bonds for the products. • The enthalpies for these two processes can then be summed up to find the total enthalpy change. • Remember: bond breaking requires energy (+ value) bond formation releases energy (- value) 12.5 Why do chemical reactions take place? Learning Objectives: 1. Explain the concept of entropy. 2. Calculate using enthalpy and entropy whether a reaction will spontaneously occur. 3. Analyse the effects of temperature on feasibility of a reaction. Is a reaction feasible or spontaneous? • Reactions that will take place on their own are called spontaneous. • If it is possible for a reaction to take place on their own, the reaction is feasible. • What determines if a reaction is feasible? • If ΔH (enthalpy) is negative, the reaction is exothermic • If ΔS (entropy) is positive, the reaction increases in randomness Entropy • Entropy is a mathematical measure of the randomness of a system. • Change in entropy is represented as ΔS. • The universe prefers randomness (higher entropy) and is always moving towards disorder. • Values for entropy of different substances are determined mathematically, you will not be expected to calculate these, only how to use them. (see pg. 179) Calculating Entropy Changes • Calculate the difference in entropy from reactants to products to find the ΔS of a reaction. ΔS = Sproducts – Sreactants • If ΔS is positive, entropy is increasing, disorder is increasing. The products are more disordered than the reactants. • If ΔS is negative, entropy is decreasing, disorder is decreasing. The products are less disordered than the reactants. Gibbs Free Energy • ΔG represents the Gibbs free energy and combines both enthalpy and entropy. • It is used to determine whether or not a reaction is feasible. ΔG = ΔH – TΔS • If ΔG is negative (-) the reaction is feasible. • If ΔG is positive (+) the reaction is NOT feasible. What happens if ΔG = 0? • There will be a temperature where ΔG = 0. • This is the temperature at which the reaction is just feasible. • In a closed system an equilibrium between products and reactants occur. • ΔG = 0 can also be used to calculate ΔS. • Cases where both forms are equally likely (ie melting point), ΔG = 0. Thermodynamics does not predict the rate of a reaction • Thermodynamics = Kinetics • Thermodynamics only predicts whether a reaction is feasible. • It DOES NOT predict how quickly the reaction may take place. • Kinetics is the branch of chemistry dealing with rate of reaction.