* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A ---> B
Survey
Document related concepts
Determination of equilibrium constants wikipedia , lookup
Electrochemistry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ene reaction wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Industrial catalysts wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Transcript
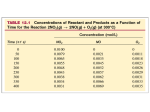
Ch 17: Kinetics Pt 1 Dr. Harris Lecture 18 HW: Ch 17: 5, 11, 18, 23, 41, 50 Reactions Rates Chemical kinetics is the area of chemistry that investigates how fast reactions occur Different reactions proceed with different rates The rate of a reaction depends on several factors, including: reactant concentration temperature catalysts surface area Today, we will focus exclusively on the relationship between reaction rates and reactant concentration Intro Lets take the reaction: A ---> B. This reaction tells us that as A is consumed, B is formed at an equal rate. We can express this mathematically in terms of changing concentrations by: − ∆𝐴 ∆[𝐵] = ∆𝑡 ∆𝑡 Imagine we have 10 moles of A in 1 L of solution. If we can freeze time for an instant, such that the reaction has not yet begin (t=0), the concentration of A is 10M. A [A] = 10 M [A] = 7 M [B] = 3 M B After 10 seconds, 3 moles of B have formed. t=0 = 1 mol of A = 1 mol of B t = 10 t = 40 40 more seconds t = 80 [A] = 3 M [B] = 7 M 10 more seconds 20 more seconds [A] = 4 M [B] = 6 M t = 20 [A] = 5 M [B] = 5 M A ---> B 10 Plotting the data from previous slide Concentration (Mol/L) 8 6 A B 4 2 0 0 10 20 30 40 Time (sec) 50 60 70 80 Reactions Follow a Rate Law The graph in the previous slide shows that the disappearance of A (formation of B) is not linear. As [A] decreases, the reaction slows down. This means that reaction rates depend on reactant concentration. This dependence of rate on concentration suggests that reaction rates follow a rate law, a mathematical expression that ties concentration and rate together Instantaneous Rates • Instantaneous rate at t=0 is the initial rate • We can determine the instantaneous rate by taking the slope of the tangent at the point of interest A ---> B 10 Concentration (Mol/L) • Although the rate of the reaction is constantly changing with reactant concentration, we can determine the instantaneous rate (reaction rate at a specific time and concentration) 8 6 A 4 2 Tangent at t = 20s, [A] = 5M 0 0 • Note: a tangent line is linear and ONLY touches the point in question. It does NOT cross the curve Instantaneous rate of disappearance of A at t=20 sec 𝑟𝑎𝑡𝑒20 = − 50 Time (sec) 7.25 − 2.30 𝑀 𝑀 = −.124 0 − 40 𝑠 𝑠 Rates and Stochiometry In the previous example (A---->B), we had 1:1 stoichiometry. Thus, at any given time, the rate of disappearance of A equals the rate of formation of B. If the stoichiometry is NOT 1:1, we have a much different situation, as shown below: 2𝐻𝐼 𝑔 → 𝐻2 𝑔 + 𝐼2 (𝑔) As you can see, 2 moles of HI are consumed for every 1 mole of H2 and 1 mole of I2 formed. Thus, the disappearance of HI is twice as fast as the appearance of the products. Example: N2O5(g) ----> 2NO2(g) + ½ O2(g) Looking at average rates average rate of disappearance after 10 minutes .0092 − .0124 𝑀 − 10 𝑚𝑖𝑛 𝑀 −𝟒 = 𝟑. 𝟐 𝒙 𝟏𝟎 𝑚𝑖𝑛 6.4 𝑥 10−4 𝑀 𝑚𝑖𝑛 10−4 𝑀 𝑚𝑖𝑛 1.6 𝑥 average rate of disappearance after 100 minutes .0006 − .0124 𝑀 − 100 𝑚𝑖𝑛 𝑀 = 𝟏. 𝟏𝟖 𝒙 𝟏𝟎−𝟒 𝑚𝑖𝑛 2.38 𝑥 10−4 𝑀 𝑚𝑖𝑛 0.59 𝑥 10−4 𝑀 𝑚𝑖𝑛 N2O5(g) ----> 2NO2(g) + ½ O2(g) slow fast Rate Laws We see that reducing reactant concentration lowers the reaction rate, but to what extent? What is the mathematical correlation? The equation that relates the concentration of the reactants to the rate of reaction is called the rate law of the reaction. We can derive the rate law of a reaction by seeing HOW THE REACTION RATE CHANGES WITH REACTANT CONCENTRATION. For any reaction aA + bB ----> cC + dD 𝑹𝒂𝒕𝒆 = 𝒌 [𝑨]𝒎 [𝑩]𝒏 In this expression, k is the rate constant, m and n are reaction orders. Reaction Orders and the Method of Initial Rates Lets go back to the previous reaction: N2O5(g) 2NO2(g) + ½ O2(g) Below is a table of data, showing the initial reaction rate as a function of the starting concentration of N2O5 (g). We perform multiple experiments to collect enough data to determine our rate law. Experiment [N2O5]o (M) Rate, M/s 1 0.010 .018 2 0.020 .036 3 0.040 .072 We see that when we double [N2O5]o, the rate also doubles. When we quadruple [N2O5]o, the rate quadruples. Thus, the rate is directly proportional to [N2O5]o by the rate constant, k. This means that the reaction is FIRST ORDER WITH RESPECT TO [N2O5] (m=1). We can write the rate law as: 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝟐𝑶𝟓]𝟏 = 𝒌[𝐍𝟐𝐎𝟓] Reaction Orders 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝟐𝑶𝟓]𝟏 = 𝒌[𝐍𝟐𝐎𝟓] Run [N2O5]o (M) Rate, M/s 1 0.010 .018 2 0.020 .036 3 0.040 .072 The overall reaction order is the sum of the individual reaction orders. In our previous example, there was only one reactant, so the overall order is 1 (1st order reaction). We can easily solve for k by plugging in any corresponding rate and concentration. Lets plug in the values from run # 1 .018 𝑀 = 𝑘(.010 𝑀) 𝑠 𝑘 = 1.8 𝑠 −1 Rate Laws/Reaction Orders 𝑹𝒂𝒕𝒆 = 𝒌 [𝑨]𝒎 [𝑩]𝒏 • Reaction orders must be determined experimentally. You can not assume based on the stoichiometry. • When you have multiple reactants, you must determine the reaction order of each one. To do this, you must vary the concentration of only one reactant at a time while holding the others fixed. • Let’s attempt to determine the rate law for the reaction below: 2NO(g) + O2(g) ---> 2NO2 Example: 2NO(g) + O2(g) ---> 2NO2 Using the data below, determine the rate law of this reaction in the form: 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶]𝒎 [𝑶𝟐 ]𝒏 Experiment [NO]o (M) [O2]o (M) Rate (M/s) 1 .0126 .0125 2.82 x 10-2 2 .0252 .0250 1.13 x 10-1 3 .0252 .0125 5.64 x 10-2 • This time, we have two reactants. Lets start by determining the value of ‘m’. To do so, we hold [O2]o fixed and vary [NO]o. This will show how the rate depends on [NO]o. • In experiments #1 and #3, [O2]o is fixed, so we will use these to find ‘m’. 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶]𝒎 [𝑶𝟐 ]𝒏 Experiment [NO]o (M) [O2]o (M) Rate (M/s) 1 .0126 .0125 2.82 x 10-2 2 .0252 .0250 1.13 x 10-1 3 .0252 .0125 5.64 x 10-2 • Remember, rate is proportional to [NO] by the power m. The factor of change in the rate is equal to the factor of change of [NO] to the mth power: order 𝑚 5.64 𝑥 10−2 .0252 = 2.82 𝑥 10−2 .0126 factor of rate change m=1 • The reaction is 1st order with respect to [NO] factor of change in [NO] 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶]𝒎 [𝑶𝟐 ]𝒏 Run [NO]o (M) [O2]o (M) Rate (M/s) 1 .0126 .0125 2.82 x 10-2 2 .0252 .0250 1.13 x 10-1 3 .0252 .0125 5.64 x 10-2 • Now we can find ‘n’ by varying [O2]o and holding [NO]o fixed. We can use experiments #2 and #3 for this. This will show how the rate depends on [O2]o. factor of rate change 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶]𝟏 [𝑶𝟐 ]𝟏 5.64 𝑥 10−2 .0125 = 1.13 𝑥 10−1 .025 n=1 𝑛 order factor of change in [O2] The reaction is 1st order with respect to [O2] and 2nd order overall. Pay Attention to the Units of k, As They Change with Overall Reaction Order The rate constant, k, is the constant of proportionality between rate and concentration. Higher values of k = faster reactions It is important to note that the units of k depend on the overall reaction order. 𝟏 𝑵𝑶− 𝟏 Ex: 𝑹𝒂𝒕𝒆 = 𝒌 𝐍𝑯+ 𝟒 𝟐 Rate is always in molarity per unit time (sec, hr, etc). Concentration is always M (mol/L). Thus, we have: 𝑀 1 =𝑘 𝑀 𝑀 →𝑘= = 𝑀 −1 𝑠 −1 𝑠 𝑀𝑠 Recall for a 1st order reaction: 𝑘 = 𝑠 −1 Units of k for a 2nd order reaction Units of k for a 1st order reaction Example 𝑹𝒂𝒕𝒆 = 𝒌 [𝑨]𝒎 [𝑩]𝒏 Determine the relative (m & n) and overall (m+n) reaction order of the reaction below. Then, derive the rate law and determine the value of k (with correct units) N𝑂2 + 𝐶𝑂 → 𝑁𝑂 + 𝐶𝑂2 Experiment [NO2]o (M) [CO]o (M) Rate (M/s) 1 .0300 .200 1 x 105 2 .0900 .200 9 x 105 3 .300 .0400 1 x 107 4 .300 .0800 1 x 107 • Tripling [NO2] causes the rate to increase nine-fold. This means that the rate is proportional to the square of [NO2], so the reaction is second order with respect to NO2 (n=2). Doubling [CO] does nothing. Thus, the rate does not depend on [CO], and is zero order with respect to CO (m=0). Overall 2nd order. 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶𝟐 ]𝟐 [𝑪𝑶]𝟎 → 𝒌 [𝑵𝑶𝟐 ]𝟐 k = 1.11 x 108 M-1s-1 Example: 2NO(g) + Br2(L) ---> 2NOBr (g) • Using the information below, determine the rate law of this reaction in the form: 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶]𝒎 [𝑩𝒓𝟐 ]𝒏 Experiment [NO]o [Br2]o Rate (M/s) 1 0.10 0.20 24 2 0.25 0.20 150 3 0.10 0.50 60 1.) Find m. We can use runs 1 & 2: 150 .25 = 24 .10 2.) Find n. We can use runs 1 & 3 60 .50 = 24 .20 𝑚 → 6.25 = 2. 5𝑚 m=2 𝑚 → 2.5 = 2.5𝑛 n=1 𝑹𝒂𝒕𝒆 = 𝒌 [𝑵𝑶]𝟐 [𝑩𝒓𝟐 ]𝟏 • The reaction is 2nd order with respect to [NO], 1st order with respect to Br2, and the reaction is overall 3rd order. Determining the Overall Rate Order of A Reaction Graphically As we have shown, a first-order reaction depends on the concentration of a single reactant to the 1st power. For the reaction: A----> products 𝑹𝒂𝒕𝒆 = 𝒌 [𝑨] Using calculus, we can convert this to: 𝐥𝐧[𝑨]𝒕 = −𝒌𝒕 + 𝐥𝐧[𝑨]𝒐 natural log of concentration at time t y axis rate constant m (slope) time x axis natural log of starting concentration b This equation is in y = mx + b form. Therefore, for any 1st order reaction, the plot of the natural log of [A]t vs time will be linear. The slope of the line will be –k. Plotting 1st Order Reactions 𝐥𝐧[𝑨]𝒕 = −𝒌𝒕 + 𝐥𝐧[𝑨]𝒐 b slope = -k units: s-1 time values on x-axis natural log of [A]t on y-axis Determining the Overall Rate Order of A Reaction graphically A second-order reaction depends on the concentration of [A] to the 2nd power. For the reaction: A ----> B 𝑹𝒂𝒕𝒆 = 𝒌 [𝑨]𝟐 y 𝟏 𝟏 = 𝒌𝒕 + [𝑨]𝒕 [𝑨]𝒐 m•x b Therefore, for any 2nd order reaction, the plot of the inverse of [A]t vs time will be linear. The slope of the line will be k. Plotting a 2nd Order Reaction 𝟏 𝟏 = 𝒌𝒕 + [𝑨]𝒕 [𝑨]𝒐 b slope = k units = M-1 s-1 time values on x-axis 1/[A]t on y-axis Determining Overall Rate Order From Plotting TimeDependent Data 𝐥𝐧[𝑨]𝒕 = −𝒌𝒕 + 𝐥𝐧[𝑨]𝒐 not linear: NOT 1st order 𝟏 𝟏 = 𝒌𝒕 + [𝑨]𝒕 [𝑨]𝒐 linear! 2nd order • We can determine if a process is first or second order by plotting the data against both equations. Which ever fitting method yields a linear plot gives the overall order.