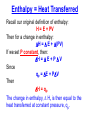* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download heat
Survey
Document related concepts
Vapor-compression refrigeration wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Dynamic insulation wikipedia , lookup
Building insulation materials wikipedia , lookup
Solar water heating wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat exchanger wikipedia , lookup
Intercooler wikipedia , lookup
Thermoregulation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
Heat equation wikipedia , lookup
Transcript
Thermochemistry (Heat of Reaction) Purpose of the Experiment Determine the heat of neutralization for the reaction of a strong acid and base; and for a weak acid with a strong base. Determine the heat of fusion of ice. What is the Heat of Reaction? Definition of Enthalpy Thermodynamic Definition of Enthalpy (H): H = E + PV E = energy of the system P = pressure of the system V = volume of the system At Constant Pressure Recall, by definition a change in energy equals heat transferred (q) plus work (w): E = q + w Consider a process carried out at constant pressure. At constant pressure, work involves only a change in volume. We can then substitute -PV for w. E = qp - PV Then if we want to solve for the heat transferred, qp, at constant pressure, we simply rearrange the equation. qp = E + PV Enthalpy = Heat Transferred Recall our original definition of enthalpy: H = E + PV Then for a change in enthalpy: H = E + (PV) If we set P constant, then: H = E + P V Since qp = E + PV Then H = qp The change in enthalpy, H, is then equal to the heat transferred at constant pressure, qp. In a chemical reaction H = H products – H reactants If H <0, then qp <0 The reaction is Exothermic. Heat goes from the system into the surroundings. An example of an exothermic reaction: If H >0, then qp >0 The reaction is Endothermic. Heat goes from the surroundings into the system. http://www.youtube.com/watch?v=rdCsbZf1_Ng Heat Capacity, C “C” is an extensive property; so a large object has a larger heat capacity than a small object made of the same material. Using the Equation: C heat absorbed q increase in tempera ture T Looking at the figures on the left, it can be seen that the temperature change is constant, but the heat absorbed by the larger object is greater. This results in a larger heat capacity for the larger object because more heat is absorbed. Specific heat capacity: The energy (joules) required to raise the temperature of 1 gram of substance by 1C Unit: J g-1K-1 or J g-1 1C-1 C Cs m Molar heat capacity: The energy (joules) required to raise the temperature of 1 mol of substance by 1C Unit: J mol-1 K-1 or J mol-1 1C-1 C Cm n Specific Heat, Cs Substance (cal/gram°C) (J/kg °C) Pure water 1.00 4,186* Wet mud 0.60 2,512 Ice (0 °C) 0.50 2,093 Sandy clay 0.33 1,381 Dry air (sea level) 0.24 1,005 Quartz sand 0.19 295 Granite 0.19 294 1 calorie = 4.186 joules *The high heat capacity of water makes it ideal for storing heat in solar heating systems. Neutralization The reaction between an acid and a base which results in a salt plus water. For example, hydrochloric acid and sodium hydroxide: HClaq + NaOHaq NaClaq + H2O acid + base salt + water Another example, cyanic acid and a hydroxide ion. If we use KOH, what salt will form? Heat of Neutralization Net ionic equation for neutralization: H+(aq) + OH-(aq) H2O(l) Energy released by reaction = Energy absorbed by solution Specific heat capacity, Cs, is defined as the quantity of heat transferred, q, divided by the mass of the substance times the change in temperature. A value of Cs is specific to the given substance. Cs = q / [(mass) (Tfinal-Tinitial)] This can then be rearranged to solve for the heat transferred. q = Cs (mass) (Tfinal-Tinitial) Enthalpy of Fusion (Melting) Enthalpy of Fusion is defined as the heat that is absorbed when the melting occurs at constant pressure. If the substance freezes, the reaction is reversed, and an equal amount of heat is given off to the surroundings; i.e., ΔHfreez = - ΔHfus solid liquid Melting (fusion) is an endothermic process H fus H m (liquid ) H m ( solid ) Calorimetry A calorimeter can be created by doing something as simple as inserting one Styrofoam cup inside another. Science of measuring heat based on observing the temperature change when a body absorbs or loses energy as heat. Calorimetry A Calorimeter may be used to determine the Heat Capacity, Cs, of a material by measuring the temperature change when a known mass of the material at a higher temperature is placed in a known mass of water, usually at room temperature, and the system is allowed to reach a final intermediate temperature. Heat lost by hot object = Heat gained by cold water Cs material (mass)material (Tfinal-Tinitial)material = Cs water (mass)water (Tfinal-Tinitial)water Note: The heat capacity is related to the atomic mass and the intermolecular forces in the material. Calorimetry A Calorimeter may be used in a similar manner to determine the enthalpy change associated with other processes, such as: Chemical reactions* (bond energies) Phase changes* (intermolecular forces) Mixing (intermolecular forces) Solvation (intermolecular forces) *These are the processes you will be learning today. Have you ever wondered about how they determine the calories in food? They use a Bomb Calorimeter. It can be used to determine the caloric value of food and of fuels, by burning them in excess oxygen and measuring the amount of heat evolved. A basic combustion reaction: CxHy + O2(excess) --> x CO2 + y/2 H2O + heat Bomb Calorimeter An example of an exothermic reaction from the S&T mining dept: http://www.youtube.com/watch?v=CIGJPWAynDQ The Computer Display Setup for Today’s Experiments 50 Temperature (oC) 40 If probe displays less than 15 oC, notify your TA. 30 20 10 0 100 200 300 400 500 600 700 800 900 1000 Tim e (s econds ) Parameters: Temperature: 10-50 oC Time: 0-1000 seconds (Check: Probe should display 15-25 oC resting on lab bench and should read higher when warmed by hand.) The Heat of Neutralization Experiments 50 Mixture not stirred fast enough – Resulting line is not vertical. Temperature (oC) 40 30 Reaction is completed, heat released, begin slow cooling to ambient 20 HCl (or acetic acid) and NaOH mixed, reaction begins 10 0 100 200 300 400 500 600 700 800 900 1000 Tim e (s econds ) Temperature change is important. Exact time is not important. Temperature will drift toward ambient before and after reaction Transition will be faster if NaOH is added rapidly and well stirred. (That is you will have a more nearly vertical temp. rise) The Heat of Fusion Experiment 50 40 Temperature (oC) Mixture not stirred fast enough – Resulting line is not vertical. Ice cube added 30 20 Melting complete, begin slow warming 10 0 100 200 300 400 500 600 700 800 900 1000 Tim e (s econds ) IMPORTANT: Use only 1 ice cube, the entire cube must melt. Checkout 1 - Calorimeter (Thermos) – Return it to the stockroom after experiment. 1 - styrofoam cup – Return it to the stockroom after experiment. Reagents in Lab _____M HCl (record concentration) _____M CH3CO2H (record concentration) _____M NaOH (record concentration) Important: Use distilled water from carboys*, NOT from the tap. (*Distilled water from the tap is normally not at room temp.) Next Week (March 12-15) – No Class (Spring Recess) Hazards HCl, strong acid, corrosive CH3CO2H, weak acid, corrosive (neutralize acid spills with solid NaHCO3) NaOH, strong base, pH>14, corrosive Waste Liquid waste labeled “Heat of Neutralization” For March 19-22 *Thermochemistry pp 11, 13, 15, & 17 and a calculations page are due. *Read over “Radiochemistry” pp 19-32 green book