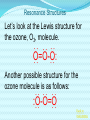* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Covalent Bonding and Nomenclature
Inorganic chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Atomic nucleus wikipedia , lookup
Coordination complex wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Electrolysis of water wikipedia , lookup
Computational chemistry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Oxidation state wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Atomic orbital wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of chemistry wikipedia , lookup
Molecular orbital wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Bent's rule wikipedia , lookup
Aromaticity wikipedia , lookup
Halogen bond wikipedia , lookup
Hydrogen atom wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Molecular dynamics wikipedia , lookup
Bond valence method wikipedia , lookup
Electron configuration wikipedia , lookup
Hydrogen bond wikipedia , lookup
Electronegativity wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Metallic bonding wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Atomic theory wikipedia , lookup
Hypervalent molecule wikipedia , lookup
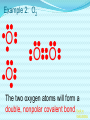
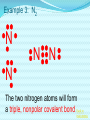
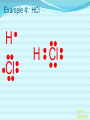
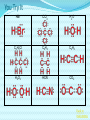
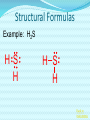
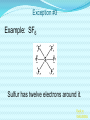
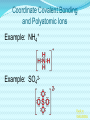
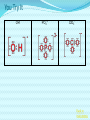
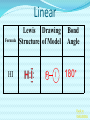
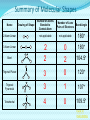
Chemical Bonding (Predicting Bond Types) Lewis (Electron) Dot Diagrams Binary Molecular Nomenclature Exceptions to the Octet Rule Coordinate Covalent Bonding Resonance Structures Molecular Shapes and Polarity Intermolecular Forces of Attraction What is a chemical bond? A chemical bond is a strong attractive force between atoms or ions in a chemical compound. Back to main menu Why do elements form chemical bonds? 1. 2. 3. Uncombined elements have relatively high potential energy. Atoms will gain, lose or share valence electrons in order to chemically combine with other atoms. By combining with other atoms, atoms decrease potential energy and create more stable arrangements. Back to main menu What two factors determine whether or not a chemical bond will form? 1. the electron configurations of the atoms involved 2. the attraction the atoms have for electrons Back to main menu How is the type of chemical bond formed between two atoms determined? The type of chemical bond formed depends upon the degree to which the valence electrons are shared between the atoms. Back to main menu Covalent Bonding In a covalent bond, valence electrons are shared by the atoms. Covalent bonds can be nonpolar or polar. Back to main menu Nonpolar vs. Polar In a nonpolar covalent bond, electrons are shared equally. Bonding which occurs between two atoms of the same element is an example of nonpolar covalent bonding. Examples: H2, Br2, O2, N2, Cl2, I2, F2 In a polar covalent bond, electrons are shared unequally. Back to main menu Ionic Bonding In an ionic bond, valence electrons are transferred between atoms. One atom gains electrons to form a negative ion (anion) and the other atom loses electrons to form a positive ion (cation). Back to main menu Ionic Bonding Which category of elements tends to gain electrons and form negative ions (anions)? nonmetals Which category of elements tends to lose electrons and form positive ions (cations)? metals Back to main menu Using differences in electronegativity to determine bond type Electronegativity is a measure of an atom’s ability to attract electrons when chemically combining with another element. The higher the electronegativity value, the stronger the attraction the atom has for another atom’s electrons. The degree to which bonding between atoms of two elements is ionic or covalent can be estimated by calculating the difference in the elements’ Back to electronegativities (ΔEN). main menu Using differences in electronegativity to determine bond type Type of Bond Nonpolar Covalent Polar Covalent Ionic ∆EN (Difference in Electronegativity) ≤0.2 0.2 to 1.7 ≥1.7 Back to main menu 1 H 2.1 3 Li 1.0 11 Na 0.9 19 K 0.8 37 Rb 0.8 55 Cs 0.7 87 Fr 0.7 Periodic Table of Electronegativities 4 Be 1.5 12 Mg 1.2 20 Ca 1.0 38 Sr 1.0 56 Ba 0.9 88 Ra 0.9 21 Sc 1.3 39 Y 1.2 57 La 1.1 89 Ac 1.1 22 23 Ti V 1.5 1.6 40 41 Zr Nb 1.4 1.6 72 73 Hf Ta 1.3 1.4 104 105 Rf Db - 24 Cr 1.6 42 Mo 1.8 74 W 1.7 106 Sg - 25 Mn 1.5 43 Tc 1.9 75 Re 1.9 107 Bh - 26 Fe 1.8 44 Ru 2.2 76 Os 2.2 108 Hs - 27 Co 1.9 45 Rh 2.2 77 Ir 2.2 109 Mt - 28 Ni 1.8 46 Pd 2.2 78 Pt 2.2 29 Cu 1.9 47 Ag 1.9 79 Au 2.4 30 Zn 1.6 48 Cd 1.7 80 Hg 1.9 110 Uun - 111 Uuu - 112 Uub - 5 B 2.0 13 Al 1.5 31 Ga 1.6 49 In 1.7 81 Tl 1.8 6 C 2.5 14 Si 1.8 32 Ge 1.8 50 Sn 1.8 82 Pb 1.8 7 N 3.0 15 P 2.1 33 As 2.0 51 Sb 1.9 83 Bi 1.9 8 O 3.5 16 S 2.5 34 Se 2.4 52 Te 2.1 84 Po 2.0 9 F 4.0 17 Cl 3.0 35 Br 2.8 53 I 2.5 85 At 2.2 113 114 Uuq - 115 116 Uuh - 117 2 He 10 Ne 18 Ar 36 Kr 3.0 54 Xe 2.6 86 Rn 2.4 118 Uuo - Back to main menu Example 1: What type of bond would form between an atom of nitrogen and an atom of chlorine? a. b. c. d. Nitrogen has an electronegativity value of 3.0. Chlorine has an electronegativity value of 3.0. The difference in the electronegativity values for nitrogen and chlorine is ΔEN = 3.0 - 3.0 = 0.0 Therefore the type of bond formed would be nonpolar covalent. The electrons would be Back to shared equally. .main menu Example 2: What type of bond would form between an atom of hydrogen and an atom of chlorine? a. b. c. d. Hydrogen has an electronegativity value of 2.1. Chlorine has an electronegativity value of 3.0. The difference in the electronegativity values for hydrogen and chlorine is ΔEN = 3.0 - 2.1 = 0.9 Therefore the type of bond formed would be polar covalent. The electrons would be Back to shared unequally. .main menu Dipole A bond formed between atoms which are not shared equally is called a dipole. a) In the bond formed between hydrogen and chlorine, the chlorine would form the negative dipole (symbolized by δ-) because it has the higher electronegativity value. b) The hydrogen would form the positive dipole (symbolized by δ+) because it has the lower electronegativity value. Back to main menu Example 3: What type of bond would form between an atom of lithium and an atom of chlorine? a. b. c. d. Lithium has an electronegativity value of 1.0. Chlorine has an electronegativity value of 3.0. The difference in the electronegativity values for lithium and chlorine is ΔEN = 3.0 - 1.0 = 2.0 Therefore the type of bond formed would be ionic. The electrons would be transferred Back to between atoms .main menu Example 3: What type of bond would form between an atom of lithium and an atom of chlorine? The lithium atom would lose electrons and form a positive ion, also known as a cation. The chlorine atom would gain electrons and form a negative ion, also known as an anion. Back to main menu You Try It 1. Complete the following table. Compound KF O2 ICl Elements Electronegativity K 0.8 F 4.0 O 3.5 O 3.5 I 2.5 Cl 3.0 ∆EN Bond Type 3.2 Ionic Nonpolar 0.0 Covalent Polar 0.5 Covalent Back to main menu You Try It 2. For each of the bonds in question 1 that were polar covalent, identify the negative dipole (δ-) and the positive dipole (δ+). ICl Iodine is the positive dipole and chlorine is the negative dipole. Back to main menu You Try It 3. Elements that exist as two atoms chemically bonded together are called diatomic elements. The diatomic elements are hydrogen, bromine, oxygen, nitrogen, chlorine, iodine, and fluorine. (You need to memorize the diatomic elements.) What type of chemical bond exists between the diatomic elements? Nonpolar covalent Back to main menu You Try It 4. Using the three classifications of bonds discussed, predict the type of bond that is most likely to be present in compounds made from elements of groups 1 (1A) and 17 (7A). Ionic Back to main menu You Try It 5. Using the three classifications of bonds discussed, predict the type of bond that is most likely to be present in compounds made from elements of groups 16 (6A) and 17 (7A). Polar Covalent Back to main menu You Try It 6. Arrange the following chemical bonds in order of least covalent to most covalent: H-H, H-Cl, H-Br, Li-Cl Li-Cl, H-Cl, H-Br, H-H Back to main menu Drawing Lewis Dot Diagrams for Atoms The electrons that play the most important role in determining whether or not a chemical bond will form are the valence electrons. In a Lewis dot diagram, dots are placed around the chemical symbol of an element to illustrate the valence electrons. The chemical symbol represents the nucleus of the atom. Back to main menu Drawing Lewis Dot Diagrams for Atoms Examples Group 1 Group 2 Group 13 Group 14 Group 15 Group 16 Group 17 H Li Group 18 He Be B C N O F Ne Back to main menu Drawing Lewis Structures for Covalent Compounds Types of Covalent Bonds Single Covalent Bond – one pair of valence electrons is shared. Double Covalent Bond - two pairs of valence electrons are shared. Triple Covalent Bond - three pairs of valence electrons are shared. Back to main menu Example 1: H2 H H H H The two hydrogen atoms will form a single, nonpolar covalent Back to bond. main menu Example 2: O2 O O O O The two oxygen atoms will form a double, nonpolar covalent bond. Back to main menu Example 3: N2 N N N N The two nitrogen atoms will form a triple, nonpolar covalent bond. Back to main menu Example 4: HCl H Cl H Cl Back to main menu Example 5: NH3 H N H H Back to main menu You Try It HBr CCl4 H 2O C2H5Cl C 2H 4 C 2H 2 H 2 O2 HCN CO2 Back to main menu Structural Formulas Structural formulas can also be used to show the arrangement of atoms in molecules. In a structural formula, dashes are used to represent shared pairs of electrons. Back to main menu Structural Formulas Example: H2 HH H–H Back to main menu Structural Formulas Example: H2S HS H H –S H Back to main menu Structural Formulas Example: N2 N N N≡ N Back to main menu Binary Molecular Nomenclature Compounds formed when atoms covalently bond are called molecular compounds. Binary molecular compounds are generally composed of two nonmetallic elements. When two nonmetallic elements combine, they often do so in more than one way. For example carbon can combine with oxygen to form carbon dioxide, CO2 and carbon monoxide, CO. Back to main menu Naming Binary Molecular Compounds Prefixes are used to show how many atoms of each element are present in each molecule of the compound. monoditritetrapenta- 1 2 3 4 5 hexaheptaoctanonadeca- 6 7 8 9 10 Back to main menu Naming Binary Molecular Compounds The names of molecular compounds have this form: (prefix + element name) (prefix + element root + ide) Back to main menu Naming Binary Molecular Compounds The prefix mono is usually omitted if there is just a single atom of the first element. Example: CO2 is carbon dioxide not monocarbon dioxide. If the vowel combinations o-o or a-o appear next to each other in the name, the first of the pair is omitted to simplify the name. Example: N2O is dinitrogen monoxide not dinitrogen monooxide. Back to main menu You Try It Name the following compounds. a. b. c. d. e. f. CBr4 Cl2O7 N2O5 BCl3 PCl5 NO a. b. c. d. Carbon tetrabromide Dichlorine heptoxide Dinitrogen pentoxide Boron trichloride e. Phosphorus pentachloride f. nitrogen monoxide Back to main menu Writing Formulas for Binary Molecular Compounds To write the formula for a binary molecular compound you simply write down the number of atoms of each element indicated by the name. Example: Carbon tetrachloride CCl4 Back to main menu You Try It Write formulas for the following binary molecular compounds. a. dinitrogen tetrahydride N2H4 b. carbon disulfide c. iodine heptafluoride CS2 IF7 d. sulfur dioxide SO2 Back to main menu Writing Formulas for Binary Molecular Compounds A few molecular compounds have common names that all scientists use in place of formal names. CH4 is methane H2O is water NH3 is ammonia You need to memorize these. Back to main menu Exceptions to the Octet Rule Some molecules are stable even though the atoms do not all obtain an octet. There are three common exceptions to the octet rule. Back to main menu Exception #1 In some molecules the central atom has less than eight valence electrons. This is called an incomplete octet. Incomplete octets are common in covalent compounds in which the central atom is beryllium, boron or aluminum. Back to main menu Exception #1 Example: BeH2 Beryllium has only four electrons around it. Back to main menu Exception #2 Molecules almost always have an even number of electrons, allowing electrons to be paired, but there are some exception in which there are an odd number of electrons. These exceptions usually involve nitrogen. Back to main menu Exception #2 Example: NO You will not be expected to draw exceptions with odd numbers of electrons in this course. Back to main menu Exception #3 In some molecules the central atom has more than eight valence electrons. This is called an expanded octet. Some common central elements that have expanded octets are sulfur, chlorine, bromine, iodine, xenon, phosphorus, and arsenic. Back to main menu Exception #3 Example: SF6 Sulfur has twelve electrons around it. Back to main menu You Try It BF3 AsH5 BeI2 PCl5 ClF5 XeF4 Back to main menu Coordinate Covalent Bonding Objectives 1. Define coordinate covalent bonding and give an example. Back to main menu Coordinate Covalent Bonding A coordinate covalent bond is formed when one atom contributes both electrons in a shared pair. Example: CO Back to main menu Coordinate Covalent Bonding and Polyatomic Ions Polyatomic ions form coordinate covalent bonds. A polyatomic ion is covalently bonded within itself, but is ionically bonded to another atom or polyatomic ion to form a neutral compound. Back to main menu Coordinate Covalent Bonding and Polyatomic Ions Example: NH4+ Example: SO42- Back to main menu You Try It OH- PO43- ClO3- Back to main menu Resonance Structures Objectives 1. Define resonance and draw resonance structures for molecules. Back to main menu Resonance Structures Resonance occurs when more than one valid Lewis structure can be written for a particular molecule. The different Lewis structures possible for a molecule are referred to as resonance structures. Back to main menu Resonance Structures Let’s look at the Lewis structure for the ozone, O3, molecule. .. .. .. O=O-O: .. .. Another possible structure for the ozone molecule is as follows: .. .. .. :O-O=O .. .. Back to main menu Resonance Structures Notice that each structure indicates that the ozone molecule has two types of O-O bonds, one single bond and one double bond. Back to main menu Resonance Structures Based on what we just learned about bond length, you would expect the bond lengths between the atoms to be different. Back to main menu Resonance Structures Scientists, however, have experimentally determined that the bond lengths between the oxygen atoms are identical. Back to main menu Resonance Structures No one structure correctly describes the ozone molecule. Scientists have determined that the structure for ozone is the average of the two structures. A double-headed arrow is used to indicate resonance. .. .. .. O=O-O: .. .. .. .. .. :O-O=O .. .. Back to main menu You Try It Resonance structures can often be written for polyatomic ions. Draw the possible resonance structures for NO2- . .. .. .. O=N-O: .. .. .. .. .. :O-N=O .. .. Back to main menu Molecular Shapes and Polarity Objectives 1. Define VSEPR and given a chemical formula of a simple molecule, identify its geometric shape as linear, trigonal planar, angular, tetrahedral, trigonal pyramidal, trigonal bypyramidal, or octahedral. 2. Using the shape of a molecule and electronegativites of its atoms, determine the polarity of the molecule. Back to main menu Molecular Shapes and Polarity The valence shell electron pair repulsion (VSEPR) theory can be used to predict the three dimensional shapes of a molecule. The main idea behind VSEPR theory is that electron pairs (bonding and nonbonding) will orient themselves so that repulsions between electron pairs are minimized. Back to main menu Linear Formula HI Lewis Drawing Structure of Model H I Bond Angle 180° Back to main menu Linear Formula HCN Lewis Drawing Structure of Model H C N Bond Angle 180° HCN is a 3-atom linear molecule. Which atom is the central atom in the HCN molecule? Carbon How many pairs of nonbonding electrons on the central Back to atom of the HCN molecule? None main menu Bent (also called angular) Formula H2O Lewis Drawing Structure of Model Bond Angle 104.5° Which atom of the water molecule is the central atom? Oxygen How many pairs of nonbonding electrons on the central atom of the H2O molecule? Two Back to main menu How can you differentiate between a linear molecule and a bent molecule in terms of nonbonding electron pairs on the central atom? Linear molecules do not have nonbonding electrons on the central atom. Bent molecules have nonbonding electrons on the central atom. Back to main menu Trigonal Planar Formula Lewis Drawing Structure of Model H2CO Bond Angle 120° Which atom is the central atom? Carbon How many atoms are bonded to the central atom? Three How many nonbonded electrons are there on the central atom? Zero Back to main menu Trigonal Pyramidal Formula Lewis Drawing Structure of Model NI3 Bond Angle 107° Which atom is the central atom? Nitrogen How many atoms are bonded to the central atom? Three How many nonbonded electrons are there on the central atom? one Back to main menu How can you differentiate between a trigonal planar molecule and a trigonal pyramidal molecule in terms of nonbonding electron pairs on the central atom? Trigonal planar molecules do not have nonbonding electrons on the central atom. Trigonal pyramidal molecules have a pair of nonbonding electrons on the central atom. Back to main menu Tetrahedral Formula Lewis Drawing Structure of Model CH4 Bond Angle 109.5° Which atom is the central atom? Carbon How many atoms are bonded to the central atom? Four How many nonbonded electrons are there on the central atom? zero Back to main menu Trigonal Bipyramidal Formula Lewis Drawing Structure of Model Bond Angle PH5 120° 90° Which atom is the central atom? Phosphorus How many atoms are bonded to the central atom? Five How many nonbonded electrons are there on the central atom? zero Back to main menu Octahedral Formula Lewis Drawing Structure of Model SH6 Bond Angle 120° 90° Which atom is the central atom? Sulfur How many atoms are bonded to the central atom? Six How many nonbonded electrons are there on the central atom? zero Back to main menu Summary of Molecular Shapes Name Drawing of Shape Number of Atoms Bonded to Central Atom Number of Lone Bond Angle Pairs of Electrons 2-Atom Linear not applicable not applicable 180° 3-Atom Linear 2 2 0 2 180° 104.5° Trigonal Planar 3 0 120° Trigonal Pyramidal 3 1 107° Tetrahedral 4 0 109.5° Bent Back to main menu Summary of Molecular Shapes Name Drawing of Shape Number of Atoms Bonded to Central Atom Number of Lone Bond Angle Pairs of Electrons Trigonal Bypryamidal 5 0 180° Octahedral 6 0 180° Back to main menu Determining Molecular Polarity The polarity of each bond, along with the geometry of the molecule, determines the polarity of the molecule. A nonpolar molecule has an even distribution of molecular charge. A polar molecule has an uneven distribution of molecular charge. Back to main menu Steps in Determining Molecular Polarity First look at the geometric shape of the molecule. Molecules with nonbonding pairs of electrons on the central atom are polar. Which two shapes are always polar? bent and trigonal pyramidal Back to main menu Steps in Determining Molecular Polarity If the molecule does contain nonbonding pairs of electrons on the central atom, the polarity is determined by the atoms surrounding the central atom. If all of the atoms surrounding the central atom are the same, the molecule is nonpolar. This is because the bond dipoles will cancel out. If all of the atoms surrounding the central atom are not alike, the molecule is polar. The bond dipoles will not cancel out. Back to main menu Steps in Determining Molecular Polarity H-C≡N: This molecule is polar. H-Be-H This molecule is nonpolar. Back to main menu You Try It Determine the polarity of each of the following molecules. a. b. c. d. e. HI H2O H2CO NI3 CH4 polar polar polar polar nonpolar Back to main menu Intermolecular Forces of Attraction Objectives 13. Define van der Waals forces, dipoledipole forces, hydrogen bonds, and London forces. 14. Given a molecule, identify the dominant type of intermolecular force of attraction. 15. Given chemical formulas for two substances, identify which type of intermolecular forces they exhibit and compare their boiling and Back to freezing points. main menu Intramolecular vs. Intermolecular Intramolecular forces – forces within a molecule that hold atoms together, that is, covalent bonds. Intermolecular forces – forces between molecules that hold molecules to each other. These intermolecular forces are collectively referred to as Van der Waals Forces. They are much weaker than covalent bonds. Back to main menu Importance of Intermolecular Forces The strength of the intermolecular forces can be used to determine whether a covalent compound exists as a solid, liquid, or gas under standard conditions. Solids have the strongest intermolecular forces of attraction between their particles. The intermolecular forces of attraction between the molecules of liquids are not as strong as those found between the particles of a solid. Gases have the weakest intermolecular forces of attraction between their particles. Back to main menu Importance of Intermolecular Forces The strength of the intermolecular forces can also be used to compare melting and boiling points. The more strongly the molecules are attracted to each other, the higher the boiling and melting points. Back to main menu Types of Intermolecular Forces London Dispersion Forces London dispersion forces exist in all covalent molecules, however; they are the most noticeable between nonpolar molecules and the nonbonding atoms of noble gases. Back to main menu Types of Intermolecular Forces London Dispersion Forces London dispersion forces arise from the motion of valence electrons. From the probability distributions of orbitals, it is concluded that the electrons are evenly distributed around the nucleus. However, at any one instant, the electron cloud may become distorted as the electrons shift to an unequal distribution. Back to main menu Types of Intermolecular Forces London Dispersion Forces It is during this instant that a molecule develops a temporary dipole. This temporary dipole introduces a similar response in neighboring molecules, thus producing a short-lived attraction between molecules. In general the larger the electron cloud, the more likely the molecule is to form temporary Back to dipoles. main menu Types of Intermolecular Forces London Dispersion Forces London forces are the weakest type of intermolecular forces of attraction. Examples: CO2, H2, Ar Back to main menu Types of Intermolecular Forces Dipole-Dipole Forces Dipole-dipole forces of attraction exist between polar molecules. Polar molecules contain uneven distributions of charge. The negative dipole of one molecule is attracted to the positive dipole of another molecule. Back to main menu Example of Dipole-Dipole Forces HCl HCl is a polar molecule. The hydrogen end of the molecule forms the positive dipole because it has the lower electronegativity. The chloride end of the molecule forms the negative dipole because it has the higher electronegativity. The chloride end of the molecule is attracted to the hydrogen end of a neighboring molecule. δ+ δ- H−Cl δ+ δ- H−Cl ↓ ↓ ↓ ↓ ↑ ↑ ↑ ↑ Cl−H δ- δ+ Dipole-dipole forces Cl−H δ- δ+ Back to main menu Types of Intermolecular Forces Dipole-Dipole Forces Dipole-dipole forces of attraction are stronger than London dispersion forces. Back to main menu Types of Intermolecular Forces Hydrogen Bonding Hydrogen Bonding is a special type of dipole-dipole force. Since no electrons are shared or transferred, hydrogen bonding is not a chemical bond. Hydrogen bonding exists between where the very electronegative elements of nitrogen, oxygen and fluorine are covalently bonded to hydrogen. Hydrogen bonding occurs between hydrogen and the unbonded electron pairs of nearby N, O, or F molecules Back to main menu Examples of Hydrogen Bonding Hydrogen bonding occurs in pure substances. The hydrogen bonding is represented by a dotted line. Back to main menu Examples of Hydrogen Bonding Hydrogen bonding can also occur in mixtures. Back to main menu Examples of Hydrogen Bonding Hydrogen bonding occurs in pure substances. The hydrogen bonding is represented by a dotted line. Back to main menu Types of Intermolecular Forces Hydrogen Bonding Hydrogen bonding is about ten times stronger than ordinary dipole-dipole forces. Back to main menu Identifying the Types of Intermolecular Forces of Attractions The chart below can help you identify the types of intermolecular forces of attraction exhibited by a substance. Reminder: London Dispersion Forces are exhibited by all covalent molecules. Back to main menu You Try It 1. List the intermolecular forces of attraction in order of increasing strength. London dispersion forces, dipole-dipole forces, hydrogen bonding Back to main menu You Try It 2. What type of intermolecular forces of attraction would be exhibited by each of the following substances? Justify your answer. The first one has been done for you. (Hint: Draw the Lewis Structure for the molecule in order to help you determine the polarity of the molecule.) a. NH3 London dispersion forces, dipole-dipole, hydrogen bonding. NH3 exhibits London dispersion forces because all covalent molecules exhibit London dispersion forces. NH3 exhibits dipole-dipole forces because it’s a polar molecule. NH3 exhibits hydrogen bonding because it’s a polar molecule in which hydrogen is bonded to a nitrogen, oxygen, or fluorine atom. In this case, hydrogen is bonded to nitrogen. Back to main menu You Try It 2. What type of intermolecular forces of attraction would be exhibited by each of the following substances? Justify your answer. The first one has been done for you. (Hint: Draw the Lewis Structure for the molecule in order to help you determine the polarity of the molecule.) b. CO2 London dispersion forces only CO2 is a nonpolar molecule. Nonpolar molecules only exhibit London dispersion forces. Back to main menu You Try It 3. What type of intermolecular forces of attraction would be exhibited by each of the following substances? Justify your answer. The first one has been done for you. (Hint: Draw the Lewis Structure for the molecule in order to help you determine the polarity of the molecule.) c. HI London dispersion forces and dipole-dipole forces HI exhibits London dispersion forces because all covalent molecules exhibit London dispersion forces. HI also exhibits dipole-dipole forces because it’s a polar molecule. It does not exhibit hydrogen bonding because since H is not bonded to O, N or F. Back to main menu You Try It 2. What type of intermolecular forces of attraction would be exhibited by each of the following substances? Justify your answer. The first one has been done for you. (Hint: Draw the Lewis Structure for the molecule in order to help you determine the polarity of the molecule.) d. NH3 London dispersion forces only BeH2 is a nonpolar molecule. Nonpolar molecules only exhibit London dispersion forces. Back to main menu Comparing Boiling Points Two factors that affect boiling point are the mass of the compound (molar mass) and the strength of the intermolecular forces of attraction. The stronger the intermolecular forces of attraction the higher the boiling point. Back to main menu Comparing Boiling Points Examine the table below. Boiling Points of Halogens Physical State at Molar Mass Boiling Point Name Formula Room (g/mol) (K, at 1 atm) Temperature gas fluorine F2 38.0 85.0 gas chlorine Cl2 70.9 239.1 liquid bromine Br2 159.8 331.9 solid iodine I2 253.8 457.4 1. What relationship exists between the mass of the halogens and the boiling point? The larger the molar mass, the higher the Back to boiling point. main menu Comparing Boiling Points Examine the table below. Boiling Points of Halogens Physical State at Molar Mass Boiling Point Name Formula Room (g/mol) (K, at 1 atm) Temperature gas fluorine F2 38.0 85.0 gas chlorine Cl2 70.9 239.1 liquid bromine Br2 159.8 331.9 solid iodine I2 253.8 457.4 2. Arrange the halogens in order of increasing intermolecular strength of attraction. Justify your answer. F2, Cl2, Br2, I2 The stronger the intermolecular forces of attraction, the greater the boiling points. Back to main menu Comparing Boiling Points 3. The graph below is a plot of the boiling points of the hydrogen compounds in the groups headed by fluorine (HF, HCl, HBr, and HI), oxygen (H2O, H2S, H2Se, H2Te), nitrogen (NH3, PH3, AsH3, SbH3), and carbon (CH4, SiH4, GeH4, SnH4). Use the graph below to answer the following questions. a. Which group of elements has the lowest boiling points for each period? Why do they have the lowest boiling points for each period? The group headed by carbon has the lowest boiling points for each period. They are all nonpolar molecules. Nonpolar molecules exhibit weaker London dispersion forces. Back to main menu Comparing Boiling Points 3. The graph below is a plot of the boiling points of the hydrogen compounds in the groups headed by fluorine (HF, HCl, HBr, and HI), oxygen (H2O, H2S, H2Se, H2Te), nitrogen (NH3, PH3, AsH3, SbH3), and carbon (CH4, SiH4, GeH4, SnH4). Use the graph below to answer the following questions. b. Notice in each of the other three groups that the first compound (H2O, NH3, and HF) in each group has a significantly higher boiling point than the other elements in their groups. What accounts for this phenomenon? H2O, NH3, and HF all exhibit hydrogen bonding. The other substances in the groups exhibit dipole-dipole forces of attraction which are not as strong as hydrogen bonding. Since H2O, NH3, and HF all exhibit hydrogen bonding they have higher than expected boiling points. Back to main menu Comparing Boiling Points 3. The graph below is a plot of the boiling points of the hydrogen compounds in the groups headed by fluorine (HF, HCl, HBr, and HI), oxygen (H2O, H2S, H2Se, H2Te), nitrogen (NH3, PH3, AsH3, SbH3), and carbon (CH4, SiH4, GeH4, SnH4). Use the graph below to answer the following questions. c. With the exception of H2O, NH3, and HF, why do the boiling points generally increase within a group? The boiling points increase because the molar mass of the compounds increases. Back to main menu You Try It 1.Determine whether each of the following would more likely be formed by polar or nonpolar molecules. a. a solid at room temperature polar b. a liquid with a high boiling point polar c. a gas at room temperature nonpolar d. a liquid with a low-boiling point nonpolar Back to main menu You Try It 2. Considering what you have learned about forces between atoms and molecules, why do you think all of the elements in group 18 exist as gases at room temperature? The noble gases exhibit London dispersion forces. London dispersion forces are the weakest of the intermolecular forces of attraction. Substances with weak intermolecular forces of attraction tend to have lower boiling points. Back to main menu You Try It 3. Arrange the following according to increasing boiling point: H2O, H2S, CO2. Justify your ranking. CO2 < H2S < H2O CO2 has only London dispersion forces. H2S has dipole-dipole forces. H2O has hydrogen bonding. Back to main menu You Try It 4. Arrange the following according to increasing boiling point: CH4, CI4, CF4. Justify your ranking. CH4 < CF4 < CI4 All three molecules are nonpolar and thus only have London dispersion forces between them. The bigger the molecule, the more electrons and thus the larger the temporary dipole. The larger the temporary dipole, the stronger the intermolecular force and thus the higher the melting point. Back to main menu You Try It 5. NH3 is a gas at room temperature and H2O is a liquid at room temperature. However, they both exhibit hydrogen bonding. What does that tell you about the strength of the hydrogen bonding in H2O as compared to NH3? The hydrogen bonding in H2O is stronger than the hydrogen bonding which occurs in NH3. H2O has two H atoms that can potentially form four hydrogen bonds with surround water molecules. There are exactly the right number of hydrogens and lone so pairs that every one of them can be involved in hydrogen bonding. In the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen has only one lone pair. Back to main menu