* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Anticoagulants Drugs
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Plateau principle wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Dydrogesterone wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
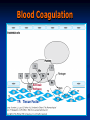
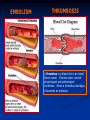
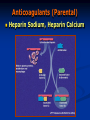
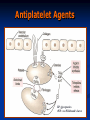
Anticoagulant, Fibrinolytic and Antiplatelet Homeostasis: Cessation of bleeding from an injured blood vessel Primary Hemostasis This stage occurs within seconds 1. Vasospasm: Vasoconstriction of the blood vessel by Prostacyclin (PI2), Thromboxane A2 (TXA2) and serotonin (5-HT). Slows down the bleeding. 2. Platelet Plug: Adherence of platelets to collagen of damaged endothelial cells. Platelet aggregation: Role of thrombin, adenosine diphosphate (ADP), PI2, TXA2, 5-HT and prostaglandins. Secondary Hemostasis This stage takes several minutes. Stabilizes the soft clot and maintains vasoconstriction. 3. Fibrin Clot: Conversion of prothrombin to thrombin. Thrombin stimulates the conversion of fibrinogen (Blood protein) to polymerized fibrin (mesh). 4. Dissolution of the clot by fibrinolysis: Plasminogen is converted to plasmin, the enzyme that dissolves the fibrin. The hemostatic process is a protective mechanism to prevent blood loss from the circulatory system. Homeostasis: Cessation of bleeding from an injured blood vessel Platelet Adhesion and Aggregation Blood Coagulation: One Type of Hemostatic Process Intrinsic Pathway Activated when the blood comes in contact with the subendothelial surface or negatively charged surface resulting from an injury to the blood vessel. The Hageman factor (factor XII) binds to the subendothelial surface. It is then cleaved to XIIa. XIIa activates XI to form XIa. XIa activates IX to form IXa which then activates factor X to Xa. Extrinsic Pathway Tissue factor (factor III) is located on the membrane of most cells. Once activated, it converts VII to its active form, VIIa. A complex of VIIa+III+calcium+a phospholipid converts factor X to its active form, Xa. Common Pathway Factor Xa is the convergence point for both the intrinsic and extrinsic pathways. It initiates the common pathway. Factor Xa converts prothrombin to active thrombin. Thrombin is required for the conversion of soluble fibrinogen to insoluble fibrin protein. The fibrin meshwork is then stabilized by active factor XIIIa. Coagulation Factors FACTOR Common Synonym Dependent on Vitamin K I Fibrinogen No II Prothrombin Yes III Tissue Thromboplastin No IV Calcium No V Proaccelerin No VII Proconvertin Yes VIII Antihemophilic Factor No IX Plasma Thromboplastin Component Yes X Stuart Factor Yes XI Plasma Thromboplastin Antecedent No XII Hageman Factor No XIII Fibrin Stabilizing Factor No Blood Coagulation Extrinsic Pathway TF: Tissue Factor Clotting Time Prothrombin Time; Prothrombin Ratio(PR) and Internationalized Ratio(INR) Measures of the extrinsic/common pathway PT reference range: 12-15 sec; measures factors II,V,VII,X and fibrinogen.; Used in conjunction with aPTT PT: blood is combined with liquid citrate (chelates calcium; anticoagulant) in a testtube. There is a specific ratio of blood to citrate. The mixture is centrifuged and the plasma is collected. Thromboplastin (Tissue factor) is added to plasma and analyzed for clotting time. INR normal range: 0.8-1.2 Activated Partial Thromboplastin Time (aPTT) Measure of the intrinsic pathway aPPT normal range: 25-37 seconds aPPT:blood is combined with liquid citrate (chelates calcium; anticoagulant) in a testtube. There is a specific ratio of blood to citrate. The mixture is centrifuged and the plasma is collected. A phospholipid (silica, kaolin, etc) and calcium are added to the plasma sample and analyzed for clotting time. Partial Thromboplastin: thromboplastin is not added to the plasma sample. EMBOLISM THROMBOSIS T H R A thrombus is a blood clot in an intact blood vessel. It forms under normal physiological and pathological conditions. When a thrombus dislodges, it becomes an embolus. Anticoagulants (Parental) Heparin Sodium, Heparin Calcium Anticoagulants (Parental) Heparin Sodium, Heparin Calcium: Unfractionated: 5000-30,000 g/mol. Naturally occurring mixture of sulfated muccopolysaccharides produced by mast cells and basophils. Clinical Use: Prevention and treatment of embolism (i.e., post-op or following myocardial infarction), deep vein thrombosis, pulmonary embolism. Initial management of unstable angina or acute myocardial infarction. MOA: Increases the activity of antithrombin III and inactivates thrombin. High doses will inhibit platelet aggregation. Pharmacokinetics: Administration: Not absorbed from the gut; i.v. and s.c.. Immediate onset (30-60 mins); Hepatic elimination and excretion, some excreted unchanged in urine. Dosage is determined by the activated partial thromboplastin time (aPPT; 1.5-2 times is normal). Side effects: Thrombocytopenia (early or delayed), hemorrhage. Contraindications: existing bleeding condition or bleeding tendency. Drug Interactions: Risk of bleeding is increased by salicylates In case of overdose: protamine sulfate (positive charge binds heparin). Anticoagulants (Parental) cont. Enoxaparin: Heparin Analog; Fractionated, Low molecular weight heparin (LMWH; 20009000 g/mol) Factor IIa is thrombin Anticoagulants (Parental) Enoxaparin: Heparin Analog; Fractionated, Low molecular weight heparin (LMWH; 2000-9000 g/mol) Clinical Use: Prevention and treatment of embolism (i.e., postop or following myocardial infarction), deep vein thrombosis. MOA: Inhibits factor Xa, very little effect on factor IIa; aPPT is not used to measure its anticoagulant activity. Binds less to plasma proteins. Pharmacokinetics: Administration: i.v. and s.c. outpatient basis for DVT patients. Immediate onset (30-60 mins); Hepatic elimination and excretion, some excreted unchanged in urine. Side effects: Thrombocytopenia (early or delayed), hemorrhage. Contraindications: existing bleeding condition or bleeding tendency. In case of overdose: protamine sulfate (positive charge binds heparin). Anticoagulants (Oral) Coumarins: dicumarol and warfarin; warfarin is structurally related to vitamin K. Clinical Use: Treatment of embolism, deep vein thrombosis or atrial MOA: Inhibits the synthesis of factors II, VII, IX and X by inhibiting the fibrillation, patients with prosthetic valves (at risk for thrombosis). production of active vitamin K. Active form Anticoagulants (Oral) Coumarins: dicumarol and warfarin; warfarin is structurally related to vitamin K. Pharmacokinetics: Route of administration: p.o.; 100% absorbed; 99% bound to plasma proteins; slow onset of activity; Hepatic elimination and excreted in the urine. Dicumarol is incompletely absorbed from the gut. Side effects: Hemorrhage. Contraindications: Patients with Hemophilia. Drug Interactions: Drugs that inhibit CytoP450 Enzymes will increase levels, ie cimetidine, Macrolide antibiotics, antifungal agents. Drugs that induce CytoP450 enzymes will decrease levels, ie rifampin and Barbituates. In case of overdose: Vitamin K (phytonadione) Fibrinolytic Drugs (Thrombolytics) These agents are enzymes or large proteins that dissolve clots, ie., an existing thrombus in pts with myocardial infarction, thrombotic stroke or pulmonary embolism. Fibrinolytic Drugs: (Thrombolytics) Tissue Plasminogen Activator (t-PA); alteplase and reteplase MOA: It is a serine protease which activates plasminogen (bound to fibrin) and increases plasmin levels. Clot specific and must bind to fibrin. 2 anti-plasmin Fibrinolytic Drugs: (Thrombolytics) Urokinase; enzyme obtained from urine MOA: Directly activates plasminogen; isolated from human kidney, therefore less chance of evoking an allergic reaction. Streptokinase: protein obtained from streptocci; anistreplase (a preformed complex of streptokinase and plasminogen) MOA: Combines with plasminogen to form an active complex that converts plasminogen to plasmin to dissolve the fibrin. Thrombolytics Pharmacokinetics: Parental administration, i,.v. Side effects: hemorrhage, hypersensitivity reactions and reperfusion arrythmias. Contraindications: Bleeding disorders; recent surgery; severe hypertension. Drug Interactions: Increases risk of bleeding with dicumarol, warfarn, heparin, aspirin, ticlopidine, abciximab. In case of overdose: Aminocaproic acid inhibits fibrinolysis by competitively blocking plasminogen activation. Antiplatelet Agents GP: glycoprotein vWF: von Willebrand’s factor Antiplatelet Agents Aspirin Clinical Use: Prevention of atherosclerosis, thrombosis, transient ischemic attacks; unstable angina. MOA: Irreversible cyclooxygenase inhibitor and inhibits the formation of thromboxane A2. Pharmacokinetics: Oral administration Side effects: Bleeding; gastrointestinal irritation, hypersensitivity reactions and thrombocypenia. Contraindications: Bleeding disorders, hypersensitivity and Reye’s syndrome. Drug Interactions: Increased hypoglycemic effects of sulfonylureas, inhibits uricosuric effect of probenecid. In case of overdose: Forced Alkaline Diuresis Antiplatelet Agents Dipyridamole Clinical Use: Prosthetic valves; may be used as an adjunct with aspirin therapy. MOA: Lowers platelet calcium and increases the formation of cAMP (weak antiplatelet drug) , coronary vasodilator. Pharmacokinetics: Oral administration Side effects: GI distress, headache, dizziness and rash. Contraindications: Hypersensitivity to this drug Drug Interactions: Increases risk of bradycardia with Beta adrenergic receptor antagonists. Antiplatelet Agents Clopidogrel (Plavix®) Clinical Use: Prevention of atherosclerosis, thrombosis, transient ischemic attacks; unstable angina. MOA: Inhibits the binding of ADP to its receptor which is involved in the activation of platelet glycoprotein receptors binding to fibrinogen. Pharmacokinetics: Oral administration; eliminated in urine and feces. Side effects: Bleeding, neutropenia and thrombocytopenia. Antiplatelet Agents Ticlopidine (Ticlid ®) Clinical Use: Patients intolerant to aspirin; prevents thrombotic stroke. MOA: Inhibits ADP-induced expression of platelet glycoprotein receptors and reduces fibrinogen binding and platelet aggregation. Effects on platelet function are irreversible. Pharmacokinetics: Oral administration; eliminated in the urine and feces Side effects: Bleeding; mild to moderate neutropenia, increased cholesterol and triglyeride levels. Contraindications: Bleeding disorders, severe liver disease Drug Interactions: Inhibits cytoP450 drug metabolizing enzymes. Antiplatelet Agents Abciximab Clinical Use: Percutaneous transluminal coronary angioplasty as adjunct with aspirin and heparin. MOA: Binds to platelet glycoprotein IIb/IIIa receptors and prevents binding to fibrinogen. Pharmacokinetics: Parental administration, i.v. Side effects: Bleeding, thrombocytopenia, hypotension and bradycardia. Contraindications: Aneurysm, bleeding, recent surgery, stroke Drug Interactions: Unknown Hemostatic Agents Aminocaprioic acid, Tranexamic acid, Antihemophilic factor, Antiinhibitor coagulants, Factor IX complex and Desmopressin Clinical Use: Decrease bleeding which may be due to hereditary deficiencies, surgery, or thrombolytic overdose.