* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Copyright © 2004 Pearson Education, Inc., publishing as Benjamin
Survey
Document related concepts
Metallic bonding wikipedia , lookup
Electrolysis of water wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Chemical bond wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Transition state theory wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Transcript
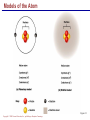
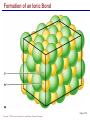
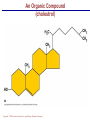
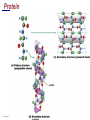
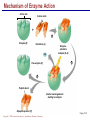
Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings A . Introduction chemistry – science that deals with the composition of substances and the Changes that take place in their composition . Organic chemistry – chemistry that deals with organic substances (those that contain carbon and hydrogen ) Biochemistry—chemistry of living organisms ; essential for understanding physiology because body functions involve chemical changes that occur within cells. Matter – anything that has weight (or mass) and takes up space .It can be solids, liquids , or gases . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Energy Energy – the ability to do work . Potential energy (PE) is stored energy in matters ; Kinetic energy (KE) is working energy produced by the motion of matters . Energy occurs in 4 forms in the human body : chemical , electrical , radiant , and mechanical energy . chemical energy is the most important form in terms of actually driving chemical reactions. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Models of the Atom Figure 2.1 Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Atomic number (AN) = number of protons = number of electrons Atomic weight (AW) = number of protons + number of neutrons Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings IONS In addition to neutrons , the electrons of atoms tend to change also – atoms that have either lost or gained electrons are called ions. Atoms that have lost electrons (as a result , now contain more p+ than e-) are called cat ions which carry positive charges , while atoms that have gained excessive electrons (as a result, now contain more e- than p+ ) are called anions which carry negative charges . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Chemically Reactive Elements Reactive elements do not have their outermost energy level fully occupied by electrons Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Figure 2.4b Bonding of atoms Ionic bonding = formed by attraction of opposite charges of a cation and an anion (e.g. Na+ + Cl- →NaCl). Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Formation of an Ionic Bond Figure 2.5b Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Covalent bonding formed by sharing of electrons between two atoms (e.g. Cl + Cl →Cl2) . The strongest type of bonding . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Hydrogen bonding formed by weak attraction between H+ and nitrogen (N) or oxygen (O) (e.g. H of a water molecule attracting to O of another water molecule). The weakest type of bonding . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Chemical reactions involve the formation , breaking , or rearrangement of chemical bonds . There are 4 general types : Dehydration synthesis : A + B → AB + water Decomposition (or hydrolysis) : AB + water → A + B Exchange : AB + CD → AD + CB Reversible : A + B < - - - > AB Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings The rate of chemical reactions is dependent on 4 factors : size of reacting molecules : smaller molecules have greater kinetic energy which produces faster reaction rate . Temperature : higher temperature creates greater kinetic energy and faster reaction rate . Concentration of reactants : higher concentration produces faster rate . Presence of catalysts : inorganic catalysts or organic catalysts (enzymes) increase reaction rate . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Electrolytes = compounds that release ions when dissolved in water (e.g. NaCl + water → Na+ + Cl- ) Acids = electrolytes that release H+ (e.g. H2 CO3 → H+ + HCO3- ) Bases = electrolytes that release anions that can combine with H+ (e.g. NaOH → Na+ + OH- ) Salts = substances formed by the reaction between an acid and a base (e.g. HCl + NaOH → H2O + NaCl ) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings PH measurement of H+ concentration in a solution - More H+ = lower PH = more acidic - Less H+ = higher pH = less acidic -Ph scale is form 0 to 14 , where the midpoint (pH 7.0) is neutral. From pH 0 to 6.9 , it is acid ; while from pH 7.1 to 14 is base . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings PH Scale Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Figure 2.9 Organic substances = chemicals that contain C and H (e.g. Carbohydrates or Protein , Fat, and nucleic acid) Inorganic substances = chemicals that do not contain C and H (e.g. table salt or NaCl , carbon dioxides or CO2 , ammonia or NH3 ) (Most inorganic substances are small, electrolytes and usually use ionic bonding , and most organic substances are large , non electrolytes, and usually use covalent bonding ). Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings An Organic Compound (cholestrol) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Protein Figure 2.3 Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Carbohydrate Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Nucleic Acid Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Solutions and concentration When a substance is dissolved in a liquid (ex. water) , a solution is formed . The substance that is dissolved is the solute and the liquid in which the dissolution occurred is the solvent . Concentration : The measure of dissolution of a particular solute in a given volume of solvent . it is measured in molarity . Molarity : The number of solute molecule per unit volume of solution . Buffer : A substance that can react with an acid or a base and thus resist a change in PH . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Tonicity the ability of a solution to change the tone or shape of cells by changing their internal H2O volume . - Hypertonic : solutions with higher osmotic pressure. cells in a Hypertonic solution lose H2O and shrink . - Hypotonic : solution with a lower osmotic pressure – cells in hyportonic solution gain H2O and swell . - Isotonic : same tonicity . cell in isotonic solutions neither gain , nor lose H2O . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings The effect of solutions of varying tonicities on red blood cell Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Enzymes 1.Are always made of globular proteins . 2.Can promote the rate of chemical reactions by billions of times. 3.Can lower the activation energy – energy necessary to start a reaction – resulting in a conservation of energy . Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings 4. Are usually reusable or recycled . 5. Are always very specific – using its active site , each enzyme is designed to bind to only one specific substance , the substrate and rapidly transforms the substrate into a product . 6. Many enzymes would not achieve their optimum efficiency unless they are bound to a cofactor (i.e. ions , metals) or to a coenzyme (organic cofactors such as vitamins ). 7. Most enzymes' names end with "ase“(ex. Dnase, Sucrase) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Mechanism of Enzyme Action Active site Amino acids 1 Enzyme (E) Substrates (s) H20 Enzymesubstrate complex (E–S) 2 Free enzyme (E) 3 Peptide bond Internal rearrangements leading to catalysis Dipeptide product (P) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Figure 2.20 Factors that affect enzyme activity Since all enzymes are made of globular proteins , and proteins are made of amino acids linked by peptide bonds , enzymes can be affected or denatured very easily . Factors that could affect or denature enzymes include heat , ration , electricity , certain chemical substances , and extreme pH. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings METABOLISM Anabolic metabolism Uses dehydration synthesis reaction to build large molecules from small molecules . Each reaction releases a water molecule and requires energy input Example – monosaccharide + energy → polysaccharide + water amino acids + energy → protein + water. Synthesis and Hydrolysis of Sucrose : Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Catabolic metabolism Uses hydrolysis (or decomposition) reaction to break up large molecules into smaller molecules . Each reaction requires a water molecule and releases energy . Example -- triglyceride + water → fatty acids + energy Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Adenosine Triphosphate (ATP) High- energy molecule that is derived from the nucleotide , adenine . Contains 3 phosphate groups (PO4) and high-energy chemical bonds that each time the bonds are broken , a large amount of energy is generated . Energy is released by ATP is broken down by hydrolysis reaction ATP + water → ADP + PO4+ energy [ADP = adenosine diphosphate ] ADP + water → AMP + PO4 + energy [AMP = adenosine monophosphate ] Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings ATP Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings