* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download atomic number.
Survey
Document related concepts
Nuclear and radiation accidents and incidents wikipedia , lookup
Radioactive decay wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear fission product wikipedia , lookup
Valley of stability wikipedia , lookup
Ionizing radiation wikipedia , lookup
Technetium-99m wikipedia , lookup
Background radiation wikipedia , lookup
Transcript
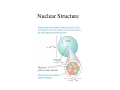
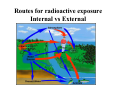
Nuclear Physics Bronze Buddha at Hiroshima The unleashed power of the atom has changed everything save our modes of thinking and we thus drift toward unparalleled catastrophe. -Albert Einstein Nuclear Power Is it Green & Safe? Nuclear Waste 250,000 tons of Spent Fuel 10,000 tons made per year Half Life used in Radiocarbon Dating Biological Effects of Ionizing Radiation Nuclear Weapons What is the World Made of? 1800’s: Spectroscopy was the Game Burning an element created a unique spectra but nobody could explain it! 1905 Einstein proves atoms exist by explaining “Brownian Motion” Born 1879 This motion makes sense if you imagine the pollen grain or dust mote being bombarded on all sides by particles too tiny to see, that are in constant motion. Einstein arrived at a mathematical explanation in terms of atoms, and integrated it into kinetic theory. 1911: Rutherford’s Planetary Model of the Atom •A beam of positively charged alpha particles hit and are scattered from a thin foil target. •Large deflections could not be explained by Thomson’s model. (Couldn’t explain spectra!) Electrons exist in quantized orbitals with energies given by multiples of Planck’s constant. Light is emitted or absorbed when an electron makes a transition between energy levels. The energy of the photon is equal to the difference in the energy levels: Eγ = Ei − E f = hf h = 6.626 x10−34 Js Characteristics of Atoms • incredibly tiny • perpetually in motion • made of a postively charged nucleus made of positively charged protons and neutral neutrons. • Electrons in shells orbiting the nucleus • Neutral atoms have equal numbers of protons and electrons. Periodic Table of the Elements Atomic Notation Atomic Mass Number A = # protons + neutrons A Z X Atomic # Neutron Number N N = # neutrons N=A-Z 1 1 H, 3 1 H, 238 92 Atomic Number Z = # protons U Why can’t we see Atoms? How do we know they exist? Molecules Molecules • consists of two or more atoms bonded together example: • H2S is hydrogen sulfide • 2 atoms of hydrogen and 1 atom of sulfur Maria Goeppert-Mayer • 1906 – 1972 • German scientist • Best known for her development of the model of the nucleus • Shared the Nobel Prize in 1963 – Shared with Hans Jensen who simultaneously developed a similar model Nuclear Structure Nucleons The nucleus is composed of two types of particles: protons and neutrons. Together, these are referred to as nucleons. The number of protons Z is the element’s atomic number. The mass number A is defined to be A = Z + N where N is the neutron number. The mass number is the total number of nucleons in a nucleus. Atomic Mass Atomic masses are specified in terms of the atomic mass unit u, defined such that the atomic mass of the isotope 12C is exactly 12 u. The conversion to SI units is 1 u = 1.6605 × 10−27 kg. The atomic mass unit can be written 1 u = 931.49 MeV/c2. It may seem unusual, but the units MeV/c2 are units of mass. Isotopes and Elements e If Helium loses a proton, it becomes a different element p n n 3H If Helium loses one of its neutrons, it becomes an isotope 3 He =T e p n p e The Hydrogen Atom • One electron orbiting a nucleus • 1 proton = Z = atomic number • 0 neutrons = N • Total mass = A = Z+N =1 p • Singly ionized Hydrogen is missing one electron = 1H+ e 1H • Add a neutron and you have Deuterium = 2H = D • Add 2 neutrons and you have Tritium = 3H = T The Helium Atom e p n n p • Two electrons orbiting a nucleus with: 2 protons = Z = atomic number 2 neutrons = N • Total mass = A = Z+N e 4He • Singly ionized Helium is missing one electron = 4He+ • Doubly ionized Helium is missing both electrons = α particle = 4He++ All Elements Have Isotopes Same # of protons - different # of neutrons Atomic Mass of an Element is an average of all Isotopes Isotopes have the same chemistry as the atom. This is why radioactive isotopes can be so dangerous. The body doesn’t see the difference between water made with hydrogen and water made with tritium. Protons repel each other! How is an Atomic Nucleus Stable? Strong Force is STRONGER than the Coulomb Force over short distances: Short Range Force FStrong ~ 100 FCoulomb Over a range of 10-15 m. Why are Atoms Not Stable? Why do Atoms Decay? As nuclear size increases, the distance between nucleons increases and the strong force becomes too weak to overcome the Coulomb electrical repulsion. The nucleus is unstable and can decay. Stable Nuclei Neutrons: Nuclear Glue With few exceptions, naturally occurring stable nuclei have N ≥ Z. For Z ≤ 20, N = Z is stable. Elements with Z ≥83 are unstable and spontaneously decay until they turn into stable lead with Z = 82. Marie Curie • 1867 – 1934 • Polish scientist • Shared Nobel Prize in 1903 for studies in radioactive substances – Prize in physics – Shared with Pierre Curie and Becquerel • Won Nobel Prize in 1911 for discovery of radium and polonium – Prize in chemistry Radioactivity • Radioactivity is the spontaneous emission of radiation – Discovered by Becquerel in 1896 – Many experiments were conducted by Becquerel and the Curies • Experiments suggested that radioactivity was the result of the decay, or disintegration, of unstable nuclei Nuclear Radiation Atomic decay by Alpha and Beta radiation causes atomic transmutation. Gamma radiation does not transmutate the atom, it changes its energy. Activity (Decay Rate) Units • The unit of activity, R, is the curie (Ci) – 1 Ci ≡ 3.7 x 1010 decays/s • The SI unit of activity is the becquerel (Bq) – 1 Bq ≡ 1 decay/s • Therefore, 1 Ci = 3.7 x 1010 Bq • The most commonly used units of activity are the millicurie and the microcurie • There have been around 2,000 nuclear test explosions • Atomic Tests released approximately 9 MCi of Sr-90 • At equilibrium with the atmosphere, a gram of carbon shows an activity of about 15 decays per minute. Conceptual Physics Fundamentals—Chapter 16 When an atom undergoes radioactive decay, it A. B. C. D. becomes an ion of the same element. becomes an isotope of the same element. turns into a completely different element. may or may not turn into a completely different element. Conceptual Physics Fundamentals—Chapter 16 When an atom undergoes radioactive decay, it A. B. C. D. becomes an ion of the same element. becomes an isotope of the same element. turns into a completely different element. may or may not turn into a completely different element. Energy Released: The Mass Defect Parent atoms have more mass than product atoms. The difference is converted to Kinetic energy of the products. E= 2 Δmc Δm = ( m parents − m products ) 1eV = 1.6 x10 −19 19 J OR 1J ~ 10 eV 1MeV = 1.6 x10 −13 J Energy released with decay of: Uranium: 25Mev ~ 4 x10-12 J Carbon: 0.016 MeV~3 x10-15 J Nuclear Decay: Fission Beta Decay Neutron Decay into a Proton (Neutron Half life ~ 12 minutes) Alpha Decay Spontaneous Fission Heavy elements FISSION into lighter elements, releasing energy in the process by E = Δmc2, where Δm is the difference in mass between the parent and products. ~ 25 MeV is released in this reaction Most of the Energy is released in the form of Kinetic Energy (heat). Alpha Decay Atomic Mass Number, A, and charge is conserved for all reactions! Beta Decay Atomic Mass Number, A, and charge is conserved for all reactions! Neutrino: Weak Force Conceptual Physics Fundamentals—Chapter 16 A certain element emits one alpha particle, and its products then emit two beta particles in succession. The atomic number of the resulting element is changed by A. B. C. D. zero. minus 1. minus 2. none of these. Conceptual Physics Fundamentals—Chapter 16 A certain element emits one alpha particle, and its products then emit two beta particles in succession. The atomic number of the resulting element is changed by A. B. C. D. zero. minus 1. minus 2. none of these. Explanation: Removal of the alpha decreases the atomic number by 2, but removal of two electrons increases it by 2, so there is no net change in atomic number. Conceptual Physics Fundamentals—Chapter 16 Which of these particles, in solitude, is quite unstable? A. B. C. D. Alpha. Beta. Proton. Neutron. Conceptual Physics Fundamentals—Chapter 16 Which of these particles, in solitude, is quite unstable? A. B. C. D. Alpha. Beta. Proton. Neutron. Conceptual Physics Fundamentals—Chapter 16 When an element ejects an alpha particle, the mass number of the resulting element A. B. C. D. reduces by 2. reduces by 4. increases by 2. increases by 4. Conceptual Physics Fundamentals—Chapter 16 When an element ejects an alpha particle, the mass number of the resulting element A. B. C. D. reduces by 2. reduces by 4. increases by 2. increases by 4. Explanation: An alpha particle (a helium nucleus) has an atomic mass of 4 (4 nucleons). So ejection of an alpha particle means a loss of 4 nucleons. Thus, the mass number of the element is lowered by 4. Fusion Light elements FUSE into larger elements, releasing energy in the process by E = mc2. There is NO Spontaneous Fusion Only in very extreme conditions like the interior of a star or in a fusion bomb or reactor can you overcome the Coulomb repulsion and force nucleons to fuse. Induced Nuclear Fission Heavy elements FISSION into lighter elements, releasing energy in the process by E = Δmc2, where Δm is the difference in mass between the parent and products. About 250 MeV is released in this reaction in the form of kinetic energy of the products. Chain Reaction – U-235 141 92 1 n + 235 U → Ba + Kr + 3 ( 92 56 36 0n) 1 0 Critical Mass – Chain Reaction Critical Mass: the minimum amount of fissionable material to produce self-sustained chain reaction, a condition called criticality. In a nuclear power plant, the critical chain reaction must control the neutron flux to avoid an exponential increase in fissions, going supercritical. In a nuclear bomb, you want a supercritical chain reaction. Fissile Material of Choice U-235 & P-239 Odd number of nucleons is easier to fission U-235: 0.231 MeV more energy than U-238 Uranium: 238U is >99% in nature 235U is ~0.7% in nature. Fuels are generally enriched to at least a few percent 235U Plutonium: 239Pu is not found in nature, it is reprocessed from nuclear power plant waste or “bred” from uranium in breeder reactors Fission & Fusion Less mass per nucleon occurs in both processes. Mass per Nucleon The smaller the mass per nucleon, the greater the binding energy. Elements fission down or fuse up to Iron, the most stable element, releasing energy by E = Δmc2. Fusion Fission Binding Energy It takes energy to break up an atom. Energy must be put into a stable system to break it apart. That energy is converted to mass by E = mc2. That energy is called the Binding Energy. Binding Energy per Nucleon The most stable atoms have the most Binding Energy per nucleon. Radioactive Atoms mutate by fission or fusion until they have maximum Binding Energy per nucleon which occurs at Iron. Fission & Fusion In either FISSION or FUSION, less stable atoms mutate to more stable atoms, releasing energy in the process by E = mc2. Radioactive Series Natural radioactivity: Unstable nuclei found in nature Artificial radioactivity: Nuclei produced in the laboratory by bombarding atoms with energetic particles in nuclear reactions. Natural Transmutation Spontaneous Fission Elements with Z ≥83 are unstable and spontaneously decay by alpha and beta radiation until they turn into stable lead with Z = 82. Note: some elements can decay by both modes. Decay Series for U-238 Decay Series of 232Th • Series starts with 232Th • Processes through a series of alpha and beta decays • The series branches at 212Bi • Ends with a stable isotope of lead, 208Pb Conceptual Physics Fundamentals—Chapter 16 When uranium-238 emits an alpha particle, uranium transforms to A. B. C. D. thorium-242. thorium-238. thorium-234. any of these thorium isotopes. Conceptual Physics Fundamentals—Chapter 16 When uranium-238 emits an alpha particle, uranium transforms to A. B. C. D. thorium-242. thorium-238. thorium-234. any of these thorium isotopes. Explanation: Emitting an alpha particle reduces the atomic mass by 4. So 238 – 4 = 234. Conceptual Physics Fundamentals—Chapter 16 Compared with the mass of a uranium atom that undergoes fission, the combined masses of the products after fission are A. B. C. D. less. more. the same. none of these. Conceptual Physics Fundamentals—Chapter 16 Compared with the mass of a uranium atom that undergoes fission, the combined masses of the products after fission are A. B. C. D. less. more. the same. none of these. Conceptual Physics Fundamentals—Chapter 16 A nucleon has a greater mass when it is A. B. C. D. inside an iron nucleus. inside a uranium nucleus. outside a nucleus. none of these. Conceptual Physics Fundamentals—Chapter 16 A nucleon has a greater mass when it is A. B. C. D. inside an iron nucleus. inside a uranium nucleus. outside a nucleus. none of these. Explanation: Work is required to pull a nucleon from the nucleus. This work increases the energy of the nucleon, which is manifested in greater mass. Conceptual Physics Fundamentals—Chapter 16 The element with the least mass per nucleon in its composition is A. B. C. D. hydrogen. helium. iron. uranium. Conceptual Physics Fundamentals—Chapter 16 The element with the least mass per nucleon in its composition is A. B. C. D. hydrogen. helium. iron. uranium. Half Life The half life of a radioactive element is the time it takes for a quantity to decay to 1/2 its original amount, N0. Half Life used in Radiocarbon Dating Carbon Dating While alive, an organic material absorbs radioactive C-14 from the atmosphere and has a fixed percent of C-14 in it with a fixed rate of radioactivity. Once the plant dies, it stops absorbing C-14 and so the radioactivity is reduced. Measuring the Activity gives a measure of the amount of C-14 remaining and thus the date when the object died. Carbon Dating Carbon-14 decays with a halflife of about 5730 years by the emission of an electron of energy 0.016 MeV. At equilibrium with the atmosphere, a gram of carbon shows an activity of about 15 decays per minute. There is 1 atom of C-14 for every 8.3x1011 atoms of C-12. C → 147 N + β − 14 6 Conceptual Physics Fundamentals—Chapter 16 The half-life of uranium-238 is 4.5 billion years. Compared with the amount of uranium-238 in Earth today, only half that amount will exist in A. B. C. D. less than 4.5 billion years. 4.5 billion years. more than 4.5 billion years. none of these. Conceptual Physics Fundamentals—Chapter 16 The half-life of uranium-238 is 4.5 billion years. Compared with the amount of uranium-238 in Earth today, only half that amount will exist in A. B. C. D. less than 4.5 billion years. 4.5 billion years. more than 4.5 billion years. none of these. Comment: Since 4.5 billion years is approximately the age of Earth, half has already decayed to lead. Conceptual Physics Fundamentals—Chapter 16 A certain isotope has a half-life of one day. This means the amount of that isotope remaining at the end of two days will be A. B. C. D. zero. one-quarter. half. the same. Conceptual Physics Fundamentals—Chapter 16 A certain isotope has a half-life of one day. This means the amount of that isotope remaining at the end of two days will be A. B. C. D. zero. one-quarter. half. the same. Biological Effects of Ionizing Radiation Energy Released: The Mass Defect Parent atoms have more mass than product atoms. The difference is converted to Kinetic energy of the products. E= 2 Δmc Δm = ( m parents − m products ) 1eV = 1.6 x10 −19 19 J OR 1J ~ 10 eV 1MeV = 1.6 x10 −13 J Energy released with decay of: Uranium: 25Mev ~ 4 x10-12 J Carbon: 0.016 MeV~3 x10-15 J Nuclear Radiation Atomic decay by Alpha and Beta radiation causes atomic transmutation. Gamma radiation does not transmutate the atom, it changes its energy. Ionizing Radiation Types Alpha particles (4He++) Beta particles (e+ and e-) Gamma-rays (γ) Neutrons (n) Ionizing Radiation: neutrons Because of their tremendous penetrating ability, neutrons can be very damaging to the human body. When neutrons strike atoms of elements that are not fissionable, they can render them radioactive by changing their atomic structure. For example, in a building near a neutron bomb explosion, the neutrons can change stable cobalt in the steel girders to cobalt 60, an emitter of highly penetrating gamma radiation. Ionizing EM Radiation: UV, Xray & Gamma Energy to ionize atom or molecule: 10-1000eV Gamma Radiation: Above a few keV are Highly Penetrating High Intensity: If you were very near a nuclear device as it exploded, an intense pulse of gamma rays would destroy the functioning of your nervous system and almost immediately afterwards cause intense heating throughout your body, sufficient to vaporize you in about a microsecond. Lower Intensity: Gamma-rays injure cells by creating highenergy electrons throughout the body, charged particles which can disrupt any chemical bond they happen to encounter as they fly along. Electrons (positirons) are produced by the photoelectric effect, compton scattering or pair-production. Gamma Ray Knife Mass Extinctions? Ionizing Radiation Effects on Cells Ionization Energy ~ few eV ~ 10-18 J α,β,γ radiation can ionize atoms which break chemical bonds and damage molecules in cells: 1. Interferes with cell reproduction 2. Destruction of cell’s function or destruction of cell itself. It has the greatest effect on cells that are rapidly reproducing because they do not have time to repair the damage: 1. Fetus, infants, children (also in animals and plants) 2. Cancerous cells Ionizing Radiation can cause cancer or kill cancer! Routes for radioactive exposure Internal vs External Testing for Ingested Contamination The most common test for exposure to radioactive material is a bioassay, usually by urinalysis. As with most cases of internal contamination, the sooner the test is taken after ingesting or inhaling the contaminant, the more accurate the results will be. Most major medical centers are capable of performing this test. Exposure vs Dose “Exposure" refers to how much radioactive material entered a person's body. Not all radiation entering the body stays there. Much of it is flushed out through breathing or along with other waste products. "Dose" refers to the amount of radioactive energy that is actually absorbed by tissues in the body. For instance, about a third of the iodine-131 entering the body is absorbed by the thyroid. Traces of it are absorbed by other body organs. The rest is flushed from the body Units of Radiation Exposure • The roentgen (R) is defined as – That amount of ionizing radiation that produces an electric charge of 3.33 x 10-10 C in 1 cm3 of air under standard conditions – Equivalently, that amount of radiation that increases the energy of 1 kg of air by 8.76 x 103J • One rad (radiation absorbed dose) – That amount of radiation that increases the energy of 1 kg of absorbing material by 1 x 10-2 More Units • The RBE (relative biological effectiveness) – The number of rads of x-radiation or gamma radiation that produces the same biological damage as 1 rad of the radiation being used – Accounts for type of particle which the rad itself does not • The rem (radiation equivalent in man) – Defined as the product of the dose in rad and the RBE factor • Dose in rem = dose in rad x RBE RBE Factors, A Sample Radiation Levels • Natural sources – rocks and soil, cosmic rays – Called background radiation – About 0.13 rem/yr • Upper limit suggested by US government – 0.50 rem/yr – Excludes background • Occupational – 5 rem/yr for whole-body radiation – Certain body parts can withstand higher levels – Ingestion or inhalation is most dangerous Radiation Levels, cont. • 50% mortality rate – About 50% of the people exposed to a dose of 400 to 500 rem will die • New SI units of radiation dosages – The gray (Gy) replaces the rad – The sievert (Sv) replaces the rem SI Units, Table Radiation Dose "Dose" the amount of radioactive energy that is actually absorbed by tissues in the body. Radiation dose unit (rad) : 1 r = 0.01 J/kg The biological effect depends on the type of radiation and body part: RBE: Relative biological effectiveness Roentgen Equivalent Man: rem = rad x RBE Type of energy of radiation X rays Gamma rays Beta rays > 30KeV Beta rays < 30 KeV Neutrons, slow Neutrons, fast Alpha Rays RBE 1 1 1 1.7 2-5 10 (body) 30 (eyes) 10-20 Dose delivered in less than one day Possible latent effects (cancer) Total Average US Background Level Radiation: 382 mrem Other Units 1 Gray (Gy) = 1 J/kg = 100 rad 1 sievert (Sv) = 100 rem Conceptual Physics Fundamentals—Chapter 16 All the radiation we normally receive annually from natural sources is a fraction of 1 rem. Lethal doses of radiation begin at A. B. C. D. 5 rems. 50 rems. 500 rems. 5000 rems. Conceptual Physics Fundamentals—Chapter 16 All the radiation we normally receive annually from natural sources is a fraction of 1 rem. Lethal doses of radiation begin at A. B. C. D. 5 rems. 50 rems. 500 rems. 5000 rems. Dose: Acute vs Chronic Dose An acute radiation dose is defined as a large dose (10 rad or greater, to the whole body) delivered during a short period of time (on the order of a few days at the most). If large enough, it may result in effects which are observable within a period of hours to weeks. A chronic dose is a relatively small amount of radiation received over a long period of time. The body is better equipped to tolerate a chronic dose than an acute dose. The body has time to repair damage because a smaller percentage of the cells need repair at any given time. The body also has time to replace dead or non-functioning cells with new, healthy cells. This is the type of dose received as occupational exposure. Immediate vs Delayed FX Immediate effects are due to an acute (short term) exposure: a large exposure that takes place over a short period of time. Delayed effects are due to latency period of cancer and disease where a health effect of radiation exposure may not become apparent for months, years or several decades after the exposure occurs. Leukemia has a latency period of 2 years, other cancers, 15 or more. If a sperm or egg are damaged then the latency period can be generations. Low Dose & Cancer •It has been estimated that the radioactive fallout from the nuclear accident at Chernobyl in 1986 will cause an increase of 17,000 cancers over the lifetime of people living in the Northern Hemisphere. •Large though this estimate seems, it is dwarfed by the 513 million cancer deaths that will occur anyway in this population. •This is why it is difficult to quantify the dangers of low doses. •Some scientists (very few) claim that low doses are GOOD for adults because they keep the cells ‘in shape’ to repair themselves. Background Radiation: ~300 mrem/year •Sources are UV radiation and cosmic particles, radium, radon, potassium 40, carbon 12 present in rocks, air, and our own body cells. •Exposure to Natural Radiation induced mutations may have contributed to our evolutionary process. Most geneticists believe that humanity has reached an evolutionary peak in beneficial mutations caused by natural radiation that the species can undergo. Thus any further mutations are detrimental, causing disease and deformity. •Although the exact percent is unknown, background radiation is thought to be responsible for a portion of all cancers and genetic disorders. Increasing the Background Nuclear Fallout: Bombs, Tests & Accidents Cs-137, Sr-90, I-131 Fallout is the descent of airborne particles of dust, debris, and radioactive substances. About 200 different substances are formed from a nuclear bomb explosion. Millions of curies of radioactivity in the form of dust and debris get carried into the upper atmosphere by the mushroom cloud. Jet stream winds can carry fallout from bomb blasts around the world within a few months. There have been around 2,000 nuclear test explosions Atomic Tests released approximately 9 MCi of Sr-90 Atmospheric Testing: 1945-1963 From 1945 to 1963 the U.S.A. conducted an extensive campaign of atmospheric nuclear tests, grouped into roughly 20 test "series." After “Starfish Prime” in 1963 the Limited Test Ban Treaty was signed and testing for the U.S., Soviet Union, and Great Britain moved underground. France continued atmospheric testing until 1974 and China did so until 1980. Testing continued until 1998 Comprehensive Test Ban Treaty. http://www.ctbto.org/specials/1945-1998-by-isao-hashimoto/ U-235 Fission Fragments Nuclear Power and Weapons Cesium 137 & Strontium 90, and Pu-239 Iodine-131 •Half Life of 8 days •Decay Mode: BETA (0.606 MeV) and Gamma (0.364 MeV) •Organ most effected: Thyroid •Iodine-131 is produced by the fission of U-235 during operation of nuclear reactors and by plutonium (or uranium) in the detonation of nuclear weapons. •Pathways: Inhalation, food chain (milk, vegetables) •Most serious fallout product from nuclear testing. Average American alive at the time received a thyroid radiation exposure of 2 – 300 rads. •Chernobyl released 83 million curies of I-131 Cesium-137 •Half Life of 30 years •Decay Mode: BETA (0.19 MeV) •Decay to Barium-137 that radiates gamma (0.6MeV) •Behaves like Potassium and is taken up by living organisms as part of fluid electrolytes. •Both internal and external hazard from cancer •Ingested, it is absorbed in the intestine, settles in muscles, excreted after a few months. •Radioactive cesium is present in soil around the world largely as a result of fallout from past atmospheric nuclear weapons test. Strontium-90 •Half Life of 28 years. •Does not occur naturally. It is a by product of fission. •Beta emitter. Decays to Yttrium-90, also a beta emitter. •Behaves like Calcium and concentrates in bone where it damages stem cells of the bone marrow critical to reproduction of cells that mediate immune function. Causes leukemia and auto-immune illnesses. •Interferes with neuron communication leading to brain damage of developing frontal cortex (dyslexia, autism) •Y-90 concentrates in the glands which controls hormonal function – interferes with estrogen and testosterone which contributes to breast and prostrate cancer, sexual organs. Strontium-90 •Large amounts of Sr-90 were produced during atmospheric nuclear weapons tests in the 1950’s and 1960’s. •Large amounts of Sr-90 was released by Chernobyl. Worldwide: •Trace levels of Sr-90 in food especially dairy products and leafy vegetables which are major sources of dietary calcium. •Every person alive today has ingested some strontium-90 Plutonium 239 •Half Life of 24,000 years. •Does not occur naturally. It is a by product of fission. •Alpha emitter (5.15 MeV) •Acts like iron and can cross the placental barrier to reach fetus. •Concentrates in testicles and ovaries. •1 pound, if uniformly distributed, could hypothetically induce lung cancer in every person on Earth. •5 metric tons of plutonium are dispersed around the Earth due to nuclear tests, bombs, satellite burn ups, fires, accidents, spill and leakages. Uranium U-238 •Half Life of 4.5 billion years. •Alpha emitter (5 MeV) •Wherever you find U-238 you will find all the 14 radioactive daughters of U 238 which emit all types of radiation upt to 100 MeV in energy. •Concentrates in bone and kidneys. •Chemically behaves like Calcium. •A severe exposure (of the order of one milligram in the kidneys) causes lesions of the tubular cells and deterioration of the kidney function. U-238 Decay Series Depleted Uranium • After isotope separation, the remaining 238U is said to be “depleted” as it is missing 235U – however, 238U is highly radioactive • Uranium is a very dense metal (1.7 x Pb), making it ideal for use in armor and shell casings • The USA used depleted Uranium weapons in the Persian Gulf War (1991), in Bosnia (1995), Kosovo (1999) and Iraq (2003), Iraq? Afghanistan? Modern Nuclear War? DU Used in Recent Wars: Balkans: 200 Tons Afghanistan: 800 Tons Gulf War 1: 350 Tons Iraq War: 200 tons??? Genetic Effects and Birth Defects Radioactive metals bind to DNA molecules. Genetic effects and birth defects due to radiation exposure occur when radiation damage to a parent's DNA code is transmitted to a child. Genetic effects caused by radiation fall into two categories: (1) effects that appear in the children of an exposed parent and (2) effects that appear in later generations. NEURAL TUBE DEFECTS The neural tube develops into the spinal cord and brain. Defects occur when the neural tube fails to close completely during the early stages of pregnancy. Why is Radiation More Damaging to Fetuses & Babies? •Cells rapidly reproduce •Damage to genes is not efficiently repaired •If cell divides a defect is multiplied. •Cellular damage can lead to greater risk of leukemia or cancer •Increased risk of premature birth, low birth weight, birth defects. In early developmental stages of both humans, fish and other wildlife when cells rapidly reproduce, damage to the genes is not efficiently repaired, so that if the cell survives and divides a defect is multiplied. Thus cellular damage can lead to a greater risk of leukemia or cancer in the new-born than in the mother, typically by anywhere from ten to a hundred times as great. depending on the stage of development. Moreover, many studies have shown that there is also an increased risk of premature birth, low birthweight and birth defects. The damage is known to involve the developing immune, hormonal and central nervous systems that often does not become apparent until many years later. Especially serious is damage to different parts of the developing brain such as the prefrontal cortex, which can result in dyslexia, autism, inability to control anger, attention deficit, and reduced cognitive ability leading to academic failure, dropout, selfish behavior, depression, suicide and murder. The reason is that neurons communicate by sending out calcium ions, so that Strontium 90 and 89 can be substituted for calcium, with devastating results due to the enormous energy with which electrons or beta rays are ejected from the nucleus in the course of the radioactive transformation from Strontiun-90 to Yttrium-90, destroying neurons in the process. Nervous System Disease High-dose exposure: cancerous and benign brain tumors in people exposed, and small brain size and mental retardation in children of women who were exposed during pregnancy. low-dose exposure: brain cancer, neurological diseases, psychological diseases, dyslexia, autism, attention deficit Thyroid Tumors, Cancer & Disease THYROID NODULES Thyroid nodules are lumps in the thyroid gland which may be benign or cancerous. "Cold nodules" are non-functioning lumps in the thyroid gland. "Hot nodules" refer to overactive thyroid lumps. HYPOTHYROIDISM Hypothyroidism is a condition caused by too little thyroid hormone in the body. Symptoms include fatigue, weight gain, intolerance to cold, decreased appetite, constipation, hoarseness, menstrual irregularities, dry skin and hair changes. Autoimmune Diseases AUTOIMMUNE DISEASE or AUTOIMMUNE DISORDER An autoimmune disease is a disease caused by the immune system attacking the cells of one's own body rather than attacking foreign cells, such as germs. AUTOIMMUNE HYPOTHYROIDISM Autoimmune hypothyroidism: An autoimmune disease that prevents the thyroid from producing enough thyroid hormone. AUTOIMMUNE THYROIDITIS Damage to the thyroid caused when the body's immune system attacks and destoys cells in the thyroid. Nuclear Workers & Brain Cancer Cumulative average whole-body doses ranged from 0.67 - 4.75 rem. There is a higher than expected number of deaths from brain cancer among the nuclear industry workers studied. While chemical exposure may contribute to the risk of cancer, the only common factor among the workers was exposure to radiation. Victor Alexander. "Brain Tumor Risk Among United States Nuclear Workers." Occupational Medicine: State of the Art Reviews. Philadelphia: Hanley and Belfus, Inc., Vol. 6, No. 4, October-December, 1991, pp. 695-714. lymphedema Lymphedema is an accumulation of lymphatic fluid in the interstitial tissue that causes swelling, most often in the arm(s) and/or leg(s), and occasionally in other parts of the body. Acquired lymphedema, can develop as a result of surgery, radiation, infection or trauma. John Smitherman US Navy "We watched the Baker shot from a ship about 19 miles away from the explosion, and mist from the mushroom fell on the deck of our ship and sand fell on our deck, little pieces of metal and rocks. We tried to wash off as much of it as we could. The mushroom cloud stayed in the air for almost two days- we could see that." Smitherman later developed lymphedema, a blockage of the lymph system that causes legs and arms to swell; he had to have both legs amputated. On September 11, 1983, he died of cancer of the colon, liver, stomach, lung and spleen. He had claimed compensation for radiation damages. The Veterans Administration turned his claim down seven times. It is still pending How do we know about the Health Effects of Nuclear Radiation? Uranium Miner "I used to go in and haul the rocks out, and I guess that's where I got hurt, because there was a lot of dust after they did the blasting and we went in right away." - Bernard Benally Red Rock Navajo Reservation, Arizona Hiroshima Nagasaki Radioactive Man Told US! Animal Testing The black star in the middle of the picture shows the tracks made by alpha rays emitted from a particle of pllutonium-239 in the lung tissue of an ape. The alpha rays do not travel very far, but once inside the body, they can penetrate more than 10,000 cells within their range. This set of alpha tracks (magnified 500 times) occurred over a 48-hour period. Human Radiation Experiments Hundreds of Secret Experiments of Radioactive Material on Humans by the DOE 1944-1960 Obtained by Citizens by the Freedom of Information Act Dr. Karl Z. Morgan, the Father of Health Physics @ Oak Ridge National Lab “There is no safe level of radiation exposure.” http://www.ctbto.org/specials/1945-1998-by-isao-hashimoto/ What is Nuclear Power? Pressurized Water Reactor (PWR) U-235 absorbs slow neutrons – they are slowed down by water – the neutrons become ‘thermalized’. Control rods absorb neutrons and moderate the chain reaction. A meltdown can happen if they fail. Basic Design of a Reactor Core • Fuel elements consist of enriched uranium • The moderator material helps to slow down the neutrons • The control rods absorb neutrons • All of these are surrounded by a radiation shield Control Rods • To control the power level, control rods are inserted into the reactor core • These rods are made of materials that are very efficient in absorbing neutrons – Cadmium is an example • By adjusting the number and position of the control rods in the reactor core, the K value can be varied and any power level can be achieved – The power level must be within the design of the reactor Reactor Safety – Containment • Radiation exposure, and its potential health risks, are controlled by three levels of containment: • Reactor vessel – Contains the fuel and radioactive fission products • Reactor building – Acts as a second containment structure should the reactor vessel rupture – Prevents radioactive material from contaminating the environment • Location – Reactor facilities are in remote locations Reactor Safety – Radioactive Materials • Disposal of waste material – Waste material contains long-lived, highly radioactive isotopes – Must be stored over long periods in ways that protect the environment – At present, the most promising solution seems to be sealing the waste in waterproof containers and burying them in deep geological repositories • Transportation of fuel and wastes – Accidents during transportation could expose the public to harmful levels of radiation – Department of Energy requires crash tests and manufacturers must demonstrate that their containers will not rupture during high speed collisions Fissile Material of Choice U-235 & P-239 Odd number of nucleons is easier to fission U-235: 0.231 MeV more energy than U-238 Uranium: 238U is >99% in nature 235U is ~0.7% in nature. Fuels are generally enriched to at least a few percent 235U Plutonium: 239Pu is not found in nature, it is reprocessed from nuclear power plant waste or “bred” from uranium in breeder reactors Cooling Towers Reactor Current Status 443 plants world wide (16% energy), 103 in the US (20% energy)