* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download amine
Survey
Document related concepts
Asymmetric induction wikipedia , lookup
Discodermolide wikipedia , lookup
Hydroformylation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
Transcript
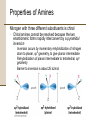
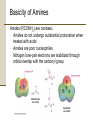
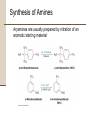
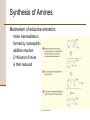
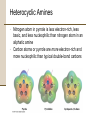
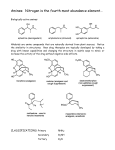
Chapter 18 Amines and Heterocycles © 2006 Thomson Higher Education Amines and Heterocycles Amines are organic derivatives of ammonia • Amines contain a nitrogen atom with a lone pair of electrons • Amines are basic and nucleophilic • Amines occur widely in both plants and animals 18.1 Naming Amines Amines can be either aklyl-substituted (alkyamines) or arylsubstituted (arylamines) • Amines are classified depending on the number of organic substituents attached to nitrogen: • Primary (RNH2) • • Secondary (R2NH) • • One organic substituent such as methylamine (CH3NH2) Two organic substituents such as dimethylamine [(CH3)2NH] Tertiary (R3N) • Three organic substituents such as trimethylamine [(CH3)3N] Naming Amines Quaternary ammonium salts • Nitrogen containing compounds with four groups attached to the nitrogen atom • Compound carry a formal positive charge Naming Amines Primary amines are named in the IUPAC system in several ways • For simple amines the suffix –amine is added to the name of the alkyl substituent • The suffix –amine can by used in place of the final –e in the name of the parent compound Naming Amines Complex amines with more than one functional group are named by considering the –NH2 as an amino substituent on the parent molecule Naming Amines Symmetrical secondary and tertiary amines are named by adding the prefix di- or tri- to the alkyl group Naming Amines Unsymmetrically substituted secondary and tertiary amines are named as N-substituted primary amines • Largest alkyl group is chosen as the parent name • Other alkyl groups are considered N-substituents on the parent • N because they are attached to nitrogen Naming Amines Heterocyclic amines • Compounds in which the nitrogen atom occurs as part of a ring • Each different heterocyclic ring system has its own parent name • The heterocyclic nitrogen atom is always numbered as position 1 18.2 Properties of Amines Nitrogen atom in alkylamines is sp3-hybridized • Three substituents occupy the three corners of a tetrahedron and the lone pair of electrons occupies the fourth corner • Bond angles are close to 109° Properties of Amines Nitrogen with three different substituents is chiral • Chiral amines cannot be resolved because the two enantiomeric forms rapidly interconvert by a pyramidal inversion • • • Inversion occurs by momentary rehybridization of nitrogen atom to planar, sp2 geometry, to give planar intermediate Rehybridization of planar intermediate to tetrahedral, sp3 geometry Barrier to inversion is about 25 kJ/mol Properties of Amines Alkylamines are starting materials for insecticides and pharmaceuticals • Labetalol is a b-blocker used for the treatment of high blood pressure • Prepared by SN2 reaction of an epoxide with a primary amine Properties of Amines Amines with fewer than five carbons are generally water-soluble • Amines form hydrogen bonds and are highly associated • H-bonding results in higher boiling points than alkanes of similar molecular weights • Amines possess characteristic odors 18.3 Basicity of Amines Chemistry of amines dominated by the lone pair of electrons on nitrogen • Lone pair makes amines both basic and nucleophilic Basicity of Amines Amines are much stronger bases than alcohols and ethers • Base strength measured by basicity constant, Kb RNH 2 + H 2O RNH 3+ + OH - RNH 3+ OH - Kb = RNH 2 pK b = - log K b Basicity of Amines Kb values are not often used • Basicity of the amine is commonly measured by determining the acidity of its conjugate acid RNH 3+ + H 2O Ka RNH 2 + H 3O + RNH 2 H 3O + RNH 3+ OH - Kb = + RNH RNH 3 2 = H 3O + OH - = K w = 1.0 10-14 Ka = and Kw Kb and Kb = pK a + pK b = 14 Kw Ka Basicity of Amines Weaker base: Smaller pKa for ammonium ion Stronger base: Larger pKa for ammonium ion Basicity of Amines Amides (RCONH2) are nonbasic • Amides do not undergo substantial protonation when treated with acids • Amides are poor nucleophiles • Nitrogen lone-pair electrons are stabilized through orbital overlap with the carbonyl group Basicity of Amines Primary and secondary amines are very weak acids • N-H proton can be removed by a sufficiently strong base • Diisopropylamine (pKa ≈ 40) reacts with butyllithium to yield lithium diisopropylamide (LDA) 18.4 Basicity of Arylamines Aryl amines are generally less basic than alkylamines • Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p electron system and less available for bonding to H+ Basicity of Arylamines Arylamines have a larger positive DG° for protonation and are therefore less basic than alkylamines, primarily because of resonance stabilization of the ground state • Nitrogen lone-pair electron density is delocalized in the amine but the charge is localized in the corresponding ammonium ion Basicity of Arylamines Electron-donating substituents which increase the reactivity for an aromatic ring toward electrophilic substitution also increase the basicity of the aryl amine Electron-withdrawing substituents which decrease ring reactivity toward electrophilic substitution also decrease arylamine basicity 18.5 Biological Amines and the Henderson-Hasselbalch Equation Amines exist essentially 100% in their protonated conjugate acid forms at physiological pH of 7.3 • Use Henderson-Hasselbalch equation to determine relative concentrations of amines and their conjugate acids A - pH = pK a + log HA A - log = pH - pK a HA Biological Amines and the Henderson-Hasselbalch Equation • For a 0.0010 M solution of methylamine at pH = 7.3 • pKa of methylammonium ion = 10.64 log CH NH 3 2 + 3 = pH - pK a = 7.3 - 10.64 CH 3 NH CH 3 NH 2 = antilog(-3.34) = 4.6 10-4 CH 3 NH 3+ so CH 3 NH 2 = 4.6 10-4 CH 3 NH 3+ and CH 3 NH 2 + CH 3 NH 3+ = 0.0010 M Solving simultaneously gives: and CH 3 NH 3+ = 0.0010M CH 3 NH 2 = 5 10-7 M Biological Amines and the Henderson-Hasselbalch Equation Cellular amines are written in their protonated forms • Amino acids shown in their ammonium carboxylate form to reflect their structures at physiological pH 18.6 Synthesis of Amines Reduction of Nitriles, Amides and Nitro Compounds • Nitriles and amides are reduced by LiAlH4 into amines • • SN2 displacement with CNfollowed by LiAlH4 reduction of the nitrile converts a primary alkyl halide into a primary alkylamine having one more carbon Amide reduction with LiAlH4 converts carboxylic acids and their derivatives into amines with the same number of carbon atoms Synthesis of Amines • Aryamines are usually prepared by nitration of an aromatic starting material Synthesis of Amines SN2 Reaction of Alkyl Halides • Ammonia and other amines are good nucleophiles in SN2 reactions • Aklylamines are synthesized most simply by SN2 alkylation of ammonia or an alkylamine with an alkyl halide Synthesis of Amines • Alkylations of ammonia and alkylamines often yield mixtures of products Synthesis of Amines Reductive Amination of Aldehydes and Ketones • Amines can be synthesized in a single step from aldehydes or ketones with ammonia in the presence of a reducing agent • Synthesis called a reductive amination Synthesis of Amines Mechanism of reductive amination • Imine intermediate is formed by nucleophilic addition reaction • C=N bond of imine is then reduced Synthesis of Amines • Ammonia, primary amines, and secondary amines can all be used in reductive amination yielding primary, secondary, and tertiary amines, respectively Synthesis of Amines • Reductive aminations occur in biological pathways • Biosynthesis of amino acid proline • Glutamate 5-semialdehyde undergoes internal imine formation to give 1-pyrrolinium-5-carboxylate • 1-pyrrolinium-5-carboxylate is reduced by nucleophilic addition of hydride ion by NADH Worked Example 18.1 Using a Reductive Amination Reaction How might you prepare N-methyl-2-phenylethylamine using a reductive amination reaction? Worked Example 18.1 Using a Reductive Amination Reaction Strategy • Look at the target molecule, and identify the groups attached to nitrogen • One of the groups must be derived from the aldehyde or ketone component and the other must be derived from the amine component • In the case of N-methyl-2-phenylethylamine there are two combinations that can lead to the product: • • Phenylacetaldehyde plus methylamine Formaldehyde plus 2-phenylethylamine • In general, it’s usually better to choose the combination with the simple amine component – methylamine in this case – and to use an excess of that amine as reactant Worked Example 18.1 Using a Reductive Amination Reaction Solution 18.7 Reactions of Amines Alkylation and Acylation • Primary and secondary (not tertiary) amines can be acylated by reaction with acid chlorides or acid anhydrides to yield amides Reactions of Amines Hofmann Elimation • Amines can be converted into alkenes by an elimination reaction • NH2 is a poor leaving group and must be converted into a better leaving group • Hofmann elimination • Amine is methylated with excess iodomethane to produce a quaternary ammonium salt • Quaternary ammonium salt undergoes elimination upon heating with silver oxide Reactions of Amines • • • • Silver oxide exchanges hydroxide ion for iodide ion in the quaternary salt Elimination is E2 Major product is the less highly substituted alkene Base abstracts hydrogen from least hindered position due to the sterically bulky trialkylamine leaving group Reactions of Amines • Biological eliminations analogous to the Hofmann elimination occur frequently • In the biosynthesis of nucleic acids adenylosuccinate undergoes elimination of a positively charged nitrogen to give fumarate plus adenosine monophosphate Reactions of Amines Electrophilic Aromatic Substitution • Amino substituents are strongly activating, ortho- and para-directing groups in electrophilic aromatic substitution • Often give polysubstituted products Reactions of Amines • Amido- substituted (-NHCOR) benzenes are less strongly activated because the nitrogen lone-pair electrons are delocalized by neighboring carbonyl group Reactions of Amines • Sulfa drugs are prepared by chlorosulfonation of acetanilide • • Reaction of p-(N-acetylamino)benzenesulfonyl chloride with ammonia gives a sulfonamide Amide can be hydrolyzed in presence of sulfonamide group 18.8 Heterocyclic Amines Pyrrole and Imidazole • Heterocyclic amines are common in biological systems Heterocyclic Amines • Most heterocycles have the same chemistry as their open-chain counterparts • • Many unsaturated ring heterocycles exhibit unique chemistry Pyrrole is an aromatic heterocycle prepared by reacting furan with ammonia over alumina Heterocyclic Amines • Each carbon of pyrrole contributes one p electron and the sp2-hybridized nitrogen contributes two from its lone pair Heterocyclic Amines • Nitrogen atom in pyrrole is less electron-rich, less basic, and less nucleophilic than nitrogen atom in an aliphatic amine • Carbon atoms or pyrrole are more electron-rich and more nucleophilic than typical double-bond carbons Heterocyclic Amines • Chemistry of pyrrole is similar to activated benzene rings • • Heterocycles are more reactive toward electrophiles than benzene rings and often require low temperatures Halogenation, nitration, sulfonation, and Friedel-Crafts acylation can all be accomplished with aromatic heterocycles Heterocyclic Amines • Electrophilic substitution normally occurs at C2 next to the nitrogen atom • Substitution at C2 gives more stable intermediate with three resonance forms Heterocyclic Amines • Imidazole (a constituent of histidine) and thiazole (on which the structure of thiamin is based) are common five-membered heterocyclic amines • Only the lone-pair electrons of nitrogen atoms that are not participating in the aromatic p system are basic Heterocyclic Amines Pyridine and Pyrimidine • The five carbon atoms and the sp2-hybridized nitrogen atom of pyridine contribute one p electron to the aromatic sextet • The lone-pair electrons of nitrogen atom occupy an sp2 orbital in the plane of the ring • Pyridine is less basic that alkylamines because the lone-pair electrons are in an sp2 orbital and are held more closely to the positively charged nucleus and thus less available for bonding Heterocyclic Amines • Pyridine does not readily undergo electrophilic aromatic substitution reactions • • Substitutions occur only slowly and usually at the C-3 of the ring Low reactivity of pyridine due to two factors: 1. Electrophile complexes in and acid-base reaction with the ring nitrogen placing a positive charge on the ring and deactivating it toward electrophilic aromatic substitution 2. Electron density of the ring is decreased by the inductively withdrawing electronegative nitrogen atom • Pyridine has substantial dipole moment (m = 2.26 D) Heterocyclic Amines • Pyrimidine is a constituent of nucleic acids • Pyrimidine is substantially less basic than pyridine due to the inductive effect of the second nitrogen atom 18.9 Fused-Ring Heterocycles Quinoline, isoquinoline, indole, and purine are common fuse-ring heterocycles • Quinoline alkaloid quinine is an antimalarial drug • The amino acid tryptophan is an indole derivative • The purine adenine is a constituent of nucleic acids Fused-Ring Heterocycles Quinoline and isoquinoline both undergo electrophilic substitutions less easily than benzene • Reaction occurs on the benzene ring Fused-Ring Heterocycles Indole has a nonbasic-pyrrole-like nitrogen and undergoes electrophilic substitution more easily than benzene • Substitution occurs at C3 of electron-rich pyrrole ring Fused-Ring Heterocycles Purine has three basic, pyridine-like nitrogens with lonepair electrons in sp2 orbitals in the plane of the ring • The remaining purine nitrogen is non-basic and pyrrole-like with its lone-pair electrons part of the aromatic p system 18.10 Spectroscopy of Amines Infrared Spectroscopy • Primary amines show a pair of bands at about 3350 and 3450 cm-1 • Secondary amines show a single band at 3350 cm-1 Spectroscopy of Amines Nuclear Magnetic Resonance Spectroscopy • Amine N-H absorptions can appear over a wide range and are best identified by adding a small amount of D2O to the sample tube • N-H is exchanged for N-D and the N-H signal disappears from the 1H NMR spectrum Spectroscopy of Amines • Hydrogens on the carbon next to nitrogen are deshielded because of the electron-withdrawing effect of the nitrogen • • Absorb at lower field than alkane hydrogens N-methyl groups are distinctive and absorb as a sharp three-hydrogen singlet at 2.2 to 2.6 d Spectroscopy of Amines • Carbons next to amine nitrogens are slightly deshielded in the 13C NMR spectrum and absorb about 20 ppm downfield from where they would otherwise absorb in an alkane of similar structure Spectroscopy of Amines Mass Spectrometry • • Nitrogen rule of mass spectrometry • A compound with an odd number of nitrogen atoms has an odd-numbered molecular weight • Nitrogen is trivalent thus requiring an odd number of hydrogen atoms Alkylamines undergo characteristic a cleavage • C-C bond nearest the nitrogen atom is broken Spectroscopy of Amines • Mass spectrum of N-ethylpropylamine has peaks at m/z = 58 and m/z = 72 corresponding to the two possible modes of a cleavage