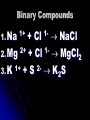* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 8
Halogen bond wikipedia , lookup
Organic chemistry wikipedia , lookup
Electric charge wikipedia , lookup
History of chemistry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Elastic recoil detection wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Cation–pi interaction wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Crystal structure wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Oxidation state wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Electronegativity wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Bond valence method wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
History of molecular theory wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Electron configuration wikipedia , lookup
Electrochemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Coordination complex wikipedia , lookup
Atomic theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Homoaromaticity wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Chemical bond wikipedia , lookup
Metallic bonding wikipedia , lookup
Chapter 8 Ionic Compounds Chemical Bonds Chemical bonds form so elements can attain a stable electron structure 8 outer electrons is the most stable structure Atoms will gain/lose electrons to reach either 0 or 8 Chemical Bonds Pseudo Noble Gas – When an ion forms that results in full s & p sub-levels (may be some d or f electrons still hanging on) Cations (Positive Ions) Results from an atom losing electrons to increase their stability Charge is equal to the number of electrons lost Sodium loses 1 electron charge = 1+ All metals Anions (Negative Ions) Formed when elements gain electrons in bonding 2. To name add –ide to element name 1. Sulfur = sulfide 3. Charge = number of electrons gained 1. Fluorine gains 1 charge = 11. Assignment Make a list of 10 elements 2. Exchange with a partner and give: 1. Electron configuration 2. Type of ion formed (cation/anion) 3. Electron dot diagram 4. Charge of the ion formed 1. Chapter 8.2 Ionic Compounds Ionic Bond Electrostatic force holds oppositely charged particles together Formed between metals and non-metals Binary Compounds Compounds that contain only two elements Metallic cation and nonmetallic anion Number of electrons lost by the cation must equal the number of electrons gained by the anion Binary Compounds 1. Na + Cl NaCl 2+ 12. Mg + Cl MgCl2 1+ 23. K + S K2S 1+ 1- Properties of Ionic Compounds During the formation of ionic compounds the positive and negative ions are arranged in repeating patterns (crystal lattice) that determine the properties of the compound as a whole Crystal Lattice 3-d geometric arrangement of particles Vary in shape due to the size and number of ions bonded Crystal Lattice Melting and Boiling points and hardness are determined by the crystal lattice Lattice energy is a result of the electrostatic attractions between oppositely charged ions There is an inverse relationship between lattice energy and interionic distance Electrolyte Ionic solution that conducts current Solid ionic compounds will not conduct since ions are not free to move Energy Changes During Bond Formation Exothermic Reaction – Energy is released during a reaction 1. Formation of ionic compounds from positive and negative ions is always exothermic 2. Endothermic Reaction – Energy is absorbed during a reaction 1. Assignment What is a chemical bond? Why do ions form? What family of elements is relatively unreactive and why? Describe the formation of both positive and negative ions What would be the ionic charge of each of the following: N S Ba Li What is an ionic bond? How does an ionic bond form List 3 physical properties of an ionic bond Describe the arrangement of ions in a crystal lattice What is lattice energy and how is it involved in an ionic bond? Explain the formation of the ionic compound for each of the following pairs: Na & N; Li & O; Sr & F; AL & S Chapter 8.3 Writing Formulas and Naming Ionic Compounds Rules for Writing Formulas for Ionic Compounds 1. 2. 3. Formula represents the simplest ratio of ions in a compound Overall charge of the compound must equal 0 Cation is always listed first Oxidation Numbers (determining charge) Monatomic Ions – Ions composed of one element 1. Oxidation number is dependent on location on the periodic table 2. If a particular oxidation is not specified use the bold oxidation number Oxidation Numbers (determining charge) Polyatomic Ions – Ions composed of more than one element, but acting as a single cation or anion 1. Table 8-6 on page 224 must be copied on your periodic table 2. Charge on table is for the entire ion 3. NEVER change subscripts in a polyatomic ion Assignment: Write the correct formula for How do you determine the correct subscripts in a the following pairs of ions chemical formula? Potassium and iodide What subscripts would most Magnesium and chloride likely be used if the following Aluminum and bromide substances formed an ionic Cesium and nitride compound? Barium and sulfide Sodium and nitrate Calcium and chlorate Aluminum and carbonate Potassium and chromate Magnesium and carbonate An alkali metal and a halogen An alkali metal and a nonmetal from group 16 An alkaline earth metal and a halogen An alkaline earth metal and a nonmetal from group 16 A metal from group 3 and a halogen Naming Ionic Compounds Oxyanions – Polyatomic ion with oxygen 1. Ion with most oxygen atoms = root of the nonmetal plus –ate 2. Ion with fewer oxygen atoms = root of nonmetal plus -ite Naming Ionic Compounds (Halogens) Most O = per- root –ate 1. ClO4 = perchlorate 2. 1 fewer = root –ate 1. ClO3 = chlorate 3. 2 fewer = root –ite 1. ClO2 = chlorite 4. 3 fewer = hypo- root –ite 1. 1. ClO = hypochlorite Binomial Nomenclature 1. 2. 3. Name cation first, anion last 1. Cation – just use the name 2. Anion – root of the name plus –ide If compound contains a polyatomic ion just name the ion Transition Metals must include a roman numeral to indicate which oxidation state is used Assignment: What is the difference between a monatomic ion • Name the following compounds: and a polyatomic ion • NaBr How are the metals, non• CaCl2 • KOH metals and polyatomic ions • CU(NO3)2 named in an ionic • Ag2CrO4 compound? What is an oxyanion and how is it named? Complete the table by providing the correct formula for each combination: Oxide Chloride Sulfate Potassium Barium Aluminum Ammonium Phosphate Chapter 8.4 Metallic Bonds/Alloys Metallic Bonding Formation of a lattice structure created when a group of atoms are crowded together and the outer-level orbitals overlap Electrons are not shared, nor are they gained or lost Metallic Bonding Electron Sea Model Electrons overlap creating an artificial stability Overlap allows electrons to move freely from one atom to another Delocalized Electrons Free to move between atoms Delocaliztion causes: High melting and boiling points Malleability and ductility Good conductivity Increase in hardness/strength with an increase in the number of electrons Alloys Mixture of metallic elements Produced to attain properties not associated with any one metal Steel = Fe & C or Ni Brass = Cu & Zn Gold Jewelry = Au & Cu Types of Alloys 1. Substitutional – Atoms of one element replace the other due to similarities in size (most common) 1. 2. 2. Sterling silver (Ag&Cu) Pewter (Sn, Sb & Pb) Interstitial – Formed when gaps between atoms are filled with smaller atoms Assignment: What is a metallic bond? Explain how the conductivity of electricity and high melting points of metals are explained by metallic bonding. What is an alloy? How does a substitutional alloy differ from an interstitial alloy? In the lab, how could you determine if a solid has an ionic bond or a metallic bond?