* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download called optics.·
Survey
Document related concepts
Optical coherence tomography wikipedia , lookup
Photoacoustic effect wikipedia , lookup
Night vision device wikipedia , lookup
Speed of light wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Bioluminescence wikipedia , lookup
Retroreflector wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Anti-reflective coating wikipedia , lookup
Nonlinear optics wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Atmospheric optics wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Opto-isolator wikipedia , lookup
Transcript
1.4 ELECTROMAGNETIC RADIATION
17
Table 1.1 shows the various kinds
of electomagnetic radiation that
have proved useful so far. Note the
very small region of the spectrum
around v = 10 14 to 10 15 cycles/sec·
or A = 10- 7 to 10- 6 m that is visi
ble light-the frequencies are com
parable to the resonance frequen-
'cycles/sec = cycles per second (cps) =
hertz. When talking of radio
frequencies. one usually uses Mc
(megacycle) = 106 cycles/sec = MHz = 106 bertz. as well as kc (kilocycle) = 103 cycles/sec = kHz = 103 hertz. Unfortunately. all these forms are used.
TABLE 1.1
·Greek ops. eye. instance. devices to generate and
detect radio waves are rather differ
ent from those for visible light or for
x-rays. Thus. for example. not only
can you take pictures using visible
light. but you can also use other
forms of electromagnetic radia
tion-gamma rays. x-rays. infrared.
etc. All you need is a source of the
radiation. some method of localiZ
ing the rays. and a detector (Fig.
1.18). The picture is a record of
how much radiation was received at
each point. The main difference be
tween different radiations is that
the smaller the wavelength. the
Electromagnetic radiation
Frequency
in hertz
Detected by
p
Wavelength in meters
Name
Examples of use
10- 15 (Size of nucleus) h
P
0
h
t
0
0
t
n
0
c
g
r
a
0
u
n
t
e
r
s
cies of the receptors of your eyes. In
this region. each frequency (or
wavelength) corresponds to a differ
ent color in the spectrum of colors
from red to violet. This tiny region
is. or course. incredibly important
to human life. The study of electro
magnetic waves in this region is
called optics.·
Much of what we will learn about
optics will be applicable to other
parts of the spectrum. although the
technology is quite different-for
P
h
1023 Gamma rays Cancer treatment
1020
p
10- 10 = 1 A (size of atom)
h
X-rays
Materials testing
Medical x-rays
0
t
C
0
c
e
1
Human
eye
s
Thermal
detectors
UltraViolet
e
m
u
1
10 - 6 = 1 ....m (diameter of
bacteria)
s
i
(UV)
Atomic structure
Germicidal
"Black light. .. sun tan
OPTICS
IR photos. heat lamps
"Heat rays." forest fire detection
Visible
0
n
Infrared
10- 2 = 1 cm (size of a
mouse)
(IR)
Microwave
Molecular structure. human body radiation
AtomiC clocks.
Space research
T
Radar. microwave ovens (3 x 109 hertz)
Radio astronomy
u
100
n
e
=
1 m (size of a man) TV. UHF: 470-890 MHz. VHF: 54-216 MHz.
FM: 88-108 MHz
International Shortwave
d
c
CB:27 MHz
Radio frequency (RF) AM radio broadcast: 550-1600 kHz
i
103
r
=
1 km (size of a Village) c
Longwave broadcast
u
i
Long-range naVigation
t
s
10" ~distance from
W' -hington. D.C.
to
10 hicago) 108 (distance to moon)
AudiO frequency
AC power.
Brain waves
CHAPTER I: FUNDAMENTAL PROPERTIES OF LIGHT
18 (a)
(b)
(e)
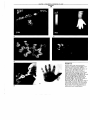
FIGURE 1.18
(e)
(d)
Pictures taken with electromagnetic
radiation of different wavelengths. (a) A
radar view of a squall line, 230 km
across. White corresponds to regions of
heaviest rain. (b) Pattern of heat
distribution seen in the far infrared
showing the heat emission from a hand
(right) and the heat image where the
hand was previously held against the wall
(left). (c) Heliotropium curassavicum
flowers are light against dark leaves in
the visible (left), but reversed in the
ultraviolet (right). Insects with vision in
the UV (see Sec. 1.4D) see them
differently than we do. (d) Action view in
x-ray "light." Exposure time was 1 J.l.sec,
and the motor turned at 116 revolutions
per second. (e) Gamma-ray picture of
human hand. Source of gamma rays is
radioactive tracer inside patient.
1.4 ELECTROMAGNETIC RADIATION
19
smaller the structures with which
they interact. For example, the an
tennas for radio waves (meters to
kilometers wavelength) are long
wires or high towers, whereas the
resonators in your eyes that select
light waves (a few hundred nano
meters in wavelength) are tiny or
ganiC molecules. Therefore, one
must be careful in taking pictures
with different wavelengths that the
wavelength is small enough to de
tect the objects of interest. If the
wavelength is comparable in size to
the object, the wave will bend
around the object (Chapter 12); if it
is much larger, the wave won't be
affected by the object. (This is why
gynecologists, bats, and whoever
else takes pictures with sound use
very short wavelengths-high fre
quencies.)
Radiation of too short a wave
length (Le., too high a frequency) is
also less useful in taking pictures.
If the radiation oscillates at too
high a frequency compared to the
resonance frequenCies of the system
of interest, the system will not be
able to keep up with the oscillations
(recall Fig. 1.12). At these high fre
quencies, then, there is very little
interaction between the radiation
and the system-the radiation just
passes through the system. This is
why many objects, such as your
skin, that are opaque to visible
light, are transparent to the higher
frequency x-rays.
B. How to make
electromagnetic radiation
Sources of radiation differ as much
as do detectors, particularly be
cause their size usually is compa
rable to a wavelength. Basically they
all are devices to wiggle charges. For
very high frequencies we try to get
nuclei and atoms to do the wiggling
for us, as these very small systems
have very high resonant frequen
Cies. At low frequenCies, we use
electronic circuits instead. One
standard way of getting radiation at
intermediate frequencies is to make
some material very hot. As we heat
it, the charges in it oscillate more
heating something means to get the
atoms in it moving more rapidly
and randomly. These wiggling
charges then radiate-the hotter
the material, the faster they wiggle,
and the higher the frequency they
radiate. The radiation from a
heated frying pan usually is not vis
ible, but you can feel it with your
hand, which responds to the in
frared radiation. If you heat the pan
suffiCiently, it begins to glow, and
becomes "red-hot," hot enough to
radiate in the visible. Your eyes are
sensitive to radiation of this higher
frequency, and you see it as red
light.
In fact, a glowing body can give
you radiation of any wavelength you
like, provided it has the proper tem
perature. For instance, your radiant
face emits radiation primarily with
wavelength near 10,000 nm. (Al
though we cannot see this radia
tion. the poisonous pit viper can.
This snake has deep hollows
pits-on the sides of its head that
are sensitive to this frequency
range. allowing it to locate its
warm-blooded victims.) Heating
and cooking goes on at about 1000
nm. and the sun's temperature cor
responds. very conveniently. to the
visible region at about 500 nm,
However. at low temperatures the
intenSity of the emitted radiation is
very low. and at high temperatures
there is plenty of intenSity but the
emitting body tends to burn. The
radiation from hot objects is not
given off at a single frequency. but
as a broad band of frequencies (Fig.
1.19). since the random heat mo
tion has no particular single fre
quency. We see from Figure 1.19
that the hotter the object. the more
light it radiates. and the shorter the
wavelength of the predominant ra
diated light. Thus. the very hot sun
radiates primarily in the visible.
whereas most of the radiation from
a 100-watt bulb is in the infrared.
with very little in the visible (mak
ing it a rather ineffiCient light
source).
The curves of Figure 1.19 are ac
tually for an ideal object called a
black bot!-y. a body that absorbs all
radiation that falls on it (Fig. 1.20).
A good approximation to a black
body is a cavity with a small en
Wavelength in nm
FIGURE 1.19
The spectrum of a glowing black body at
temperatures of a light bulb, of a
photoflood bulb, and of the sun. The
actual spectra of these radiators are only
a little different from this idealized black
body approximation. (Remember,
shorter wavelength means higher
frequency.)
trance hole. Light that enters the
cavity through this hole bounces
around inside but. like a lobster in
a lobster trap. rarely finds the hole
again. so it does not get out. Your
eye is a reasonably good black body
because it contains a small opening
with a big empty space behind it
(Sec. 5.IA),
When a black body is heated it
starts to glow. and. therefore. no
longer looks black, But it continues
to be black in the sense that it still
absorbs all radiation that falls on it.
A small window in a furnace is a
good example of a glowing black
body. If you try to illuminate this
"body" (which is really the Window
hole) with a flashlight. it will look
no different in color or brightness-
it absorbs all the light you shine on
it. Because the radiation stays in
side the furnace for a relatively long
time before it manages to escape, it
comes to equilibrium with the fur
nace. and the color emitted de
pends only on the temperature.
(Temperatures of the molten metal
in foundries, and of ceramic kilns.
CHAPTER 1: FUNDAMENTAL PROPERTIES OF LIGHT
20
G
FIGURE 1.20
A good approximation to a black body
radiator: a heated cavity with a small exit
hole (furnace in a steel mill).
are often measured by examining
the glowing color.) Most glowing
bodies are not truly black bodies.
For example, a log glowing in a fire
place Is nearly, but not quite, black,
as you can check after it has cooled.
You can verify its near blackness by
shining a light at the glowing coals
in your fireplace. (If the coals were
really black bodies at uniform tem
perature, they would all appear
equally bright. Inside a well-stoked
furnace you see a uniform bright
glow and cannot distinguish the in
dividual coals.) Similarly, the sun
and light bulbs are not truly black
bodies, though the essential fea
tures of the light they radiate are
not very different from the black
body case.
Finally, we must mention that we
cannot fully explain the curves of
Figure 1. 19 from our understand
ing of light as a wave. In fact, these
curves led to our knowledge that
the wave picture is not the entire
story (Sec. 15.2A).
c. Light sources
The fact that hot bodies glow, or in
candesce,· has played a major role
in the history of artificial light
sources. From the time Prometheus
brought fire down from Olympus
untU this century, all man-made
light sources have been incandes
cent (except for those created by
collecting fireflies). Until the advent
of adequate generators of electricity
in the nineteenth century, these
lights were very little more than the
fire Prometheus gave us; the only
innovations being improvements in
fuel from the campfires and torches
that formed the earliest light
sources.
The next known light source was
the oil lamp, used by PaleolithiC
cavedwellers to allow them to make
those magnificent cave paintings.
The oil lamp was a dish of stone,
shell, or, later, pottery that con
tained oil and a reed wick. (In the
nineteenth century, when kerosene
replaced the oil and an air draft was
introduced through the wick, the
oil lamp began to evolve into the
-Latin tn-candescere, to become white.
lantern we now take on camping
trips.)
Several millennia after the first oil
lamp, in Egypt or PhoeniCia, a can
dle was made by Impregnating fi
brous material with wax (from in
sects or certain trees). The use of
tallow, and much later spermaceti
(from sperm whales), and improve
ments of the wick, provided the ma
jor changes in this light source.
There were various civic lighting
projects. Main streets in towns had
previously been lit only by lamps in
shops, house entrances, shrines,
temples, and tombs. In the larger
towns this may have been signifi
cant. It is estimated that there was
a lamp every one to two meters
along the main streets of Pompeii.
Around 450 A.D. the first street
lights (in the form of tarred torches)
were Introduced in Antioch. Light
houses, primarily to mark the en
trances to ports, were another big
project. Originally simple hilltop
fires, these evolved into towers with
bonfires of resinous wood, later
coal, and, in the late eighteenth
century, candles and whale 011
lamps. The first known attempt to
concentrate the light from these
fires was probably the great pharos
of Alexandria (one of the seven won
ders of the ancient world, designed
by Sostratus of Cnidos in about 280
B.C.), which may have used large
mirrors of polished metal and
whose light was said to be visible
for 35 miles.
Except for a few oddities (the
most bizarre being the burning of
fatty animals, such as the candle
fish or the stormy petrel), the torch,
the oil lamp, and the candle gave
the world the bulk of its nighttime
light untU the nineteenth century.
Thus, when the sun went down, it
was very dark-". . . the night
cometh, when no man can work"
(John 9:4). Well Into the nineteenth
century battles stopped at sun
down, which was all to the good.
but hospital care ceased also.
Witches had to wait for a full moon
for a nighttime ramble; the poor
went to bed at sunset; only the rich
had a nightlife. About a century
ago, this began to change with
new lighting innovations (a mixed
1.4 ELECTROMAGNETIC RADIATION
21
blessing-they brought the 12-hour
workday).
The nineteenth century intro
duced gas lighting. While the
Chinese had burned natural gas
(piping it from salt mines through
bamboo tubes), and coal gas was
distilled from coal in 1664, gas was
not used very much until it became
economically attractive. around
1800. Introducing air. or oxygen.
with the gas was found to improve
the light. An even brighter light
was produced by heating a block
of lime to incandescence in an oxy
hydrogen flame. producing the
limelight. which was used for
"magic lanterns." and soon after
mid-century for the theatrical appli
cations that preserve its name to
day. (The explosive nature of the
gas. combined with the flammable
scenery. made theatergoing some
what more of an adventure then.)
Introduction in 1885 of the gas
mantle. a mesh of inorganic salts
heated to incandescence. increased
the light by a factor of six over that
obtained by just burning the gas.
and kept the gas light industry alive
into this century. (The improve
ment is a result of the fact that
these salts are not ideal black bod
ies. Rather they tend to emit some
what more in the visible. thus mak
ing them more effiCient in pro
dUCing useful light. The purpose of
the gas. then. is just to heat the
mantle. so the gas can be less lu
minous and less smoky.)
The earliest electric light was the
arc lamp in which a spark jumped
across two electrodes attached to a
large battery. Arc lamps of any prac
tical value had to await the devel
opment of big electrical generators
in the mid-1800s. and shortly
thereafter brilliant arc lights were
in wide use. In these lamps. an elec
tric field is set up between two hot
electrodes (here. carbon rods) that
are separated by a narrow gap (Fig.
1.21). Electrons are emitted from
one electrode. pulled across the gap
by the electric field. smash into the
air molecules in between. knock
electrons off them. and create many
charged atoms (ions), which in tum
are also accelerated by the electriC
field. All these charges smash into
FIGURE 1.21
An AC carbon arc. Only one of the
carbon electrodes would glow if the arc
were fed DC, as in the earliest arc lights.
Most of the light comes from the very
hot electrode tips.
each other and into the electrodes.
give off 11ght. and further heat the
carbon electrodes. In fact. over 90%
of the light comes from the incan
descence of these very hot electrode
tips. This produces a very concen
trated light. While it is much too
bright a light for the home. the car
bon arc still is found in theatrical
spotlights.
The incandescent filament lamp
used today had to await the devel
opment of the mercury vacuum
pump in order to produce the
needed quality vacuum. The pump
was produced in 1865 and by 1880
Edison had his patent for the in
candescent light (Fig. 1.22). En
closed within a glass bulb there is a
little coil of thin wire. thejUam.ent,
made of tungsten. because tung
sten can become very hot without
melting. The filament is about half
a meter long if unwound, and its
ends are connected to the lamp-
cord. As the current runs through
it. the filament becomes hot and ra
diates. Some radiation is in the vis
ible (Fig. 1.19) but most is in the
infrared! You have undoubtedly felt
that light bulbs get hot. In fact an
incandescent bulb is only about 7%
effiCient in converting electricity to
visible light-the rest of the energy
goes into heat. We could get proporFilament
Support wires
Base
FIGURE 1.22
CHAPTER 1: FUNDAMENTAL PROPERTIES OF LIGHT
22
tionately more visible radiation if
we heated the filament more, but
the tungsten would then melt or
bum. To prevent such burning
some of the air is pumped out of the
glass bulb. (That's why the bulb
goes "pop" when it breaks, and why
they needed the vacuum pump.) Ac-
tually, there is only a partial vac
uum in the bulb. It is filled with a
mixture of gases (argon and nitro
gen) that do not react appreciably
with tungsten. These gases tend to
retard evaporation of the tungsten.
The evaporated tungsten deposits
on the glass (you can see this dark
ening in an old bulb) and makes the
bulb dimmer, and the filament de
velops mechanically weak, hot
spots. When the filament finally
breaks, the bulb is "burnt out";
current can no longer flow.
In newer lamps, called "quartz
halogen" or "quartz-iodine" or
"tungsten-halogen" lamps, the fila
ment is surrounded by a quartz en
closure containing a Uttle iodine
gas (Fig. 1.23). The iodine picks up
any tungsten that has been evapo
rated and redeposits it on the fila
ment. This allows the lamp to oper
ate at a higher temperature, which
makes it more efficient since you
get more visible Ught and propor
tionately less heat. Further, the
evaporated filament doesn't blacken
the bulb.
The relatively low efficiency of all
incandescent sources led, this cen
tury, to the development ofjluores
cent lamps,· the first departure
from the long historical precedent
of producing light by heating some
thing. In jluorescence, certain
substances (called phosphors) pro
duce visible light when they absorb
ultraviolet ("black") light, and thus
make light without using heat. Sur
prisingly, ultraviolet radiation can
be more easily produced effiCiently.
A glass tube is filled with some gas
at low pressure, usually mercury va
por (Fig. 1.24). Electrodes at the
*Latinfluere. to flow. But not of
current: fluorescence was first observed
in the mineral fluorite (CaF2 1. which
was used as a flowing agent to help
metals fuse together when melted.
FIGURE 1.23
Tungsten-halogen lamp.
FIGURE 1.24
A fluorescent tube first makes UV light in
an electric gas discharge and then
converts most of the UV to visible light.
Electrode
end are connected to an alternatiIlj
current (AC) source. which drive
the charges first one way and thel
the opposite way. (In the Unite.
States. alternating currents g.
through a complete cycle 60 times;
second. so we have 60 hertz AC.
The resulting electric field in th,
tube pulls some electrons off th
electrodes. These electrons collid
with atoms. shaking them and th
charges on them. The whole pro
cess is called a discharge. The os
cillation of charges on atoms occur,
at the atoms' resonance frequency
and this frequency for simple atom:
Is mainly in the ultraviolet (wit]
some in the visible).
To make a "black light." you coa
the tube with material that absorb:
the visible but transmits the ultra
violet (UV). On the other hand, t.
make visible light. the tube i:
coated instead with material tha
fluoresces. This way of making vis
ible Ught is so efficient that a 40
watt fluorescent lamp provide:
about 4 times as much visible Ugh
as a 40-watt incandescent bulb
The fluorescent lamp is mud
cooler. hence wastes less energy ra
diating infrared. Further. by choos
ing different coating materials. ym
can have fluorescent bulbs that giv,
off different colors. Thus. specia
lamps for growing plants indoor:
use a phosphor chosen to give I
spectrum of light similar to sun
Ught. or to match the resonance fre
quencies of chlorophyll.
We could make a more efficielli
Ught by using the Ught from tht
discharge directly. without using c
phosphor. The Ught from a dis
charge. however. has a frequenc)
(and thus color) characteristic 01
the particular resonant system,
here the atoms in the gas (see Secs.
15.3 and 15.4). To make a useful
Atom
@0 0-
Phosphor coating
Visible
1.4 ELECTROMAGNETIC RADIATION
23
charged particles from the sun
strike molecules in our atmosphere.
The radiation produced depends on
the energy to which the charges are
accelerated by the earth's magnetic
and electric fields, as well as on the
molecules they hit.
light, we choose atoms that have
resonances in the visible, for exam
ple, mercury or sodium. The strong
coloration of the light emitted by
these atoms is avoided in high-in
tensity discharge lamps, where
high
pressure
or
impurities
broaden the range of frequencies
emitted, making a light somewhat
more like broadband white.
The production of light due to
collisions of accelerated charged
particles with atoms is not only a
man-made phenomenon. The glow
ing aurora borealis is essential
ly the same phenomenon. Here
......1 - - - -
Frequency
/I
D. Visible electromagnetic
radiation
Well concentrate now, and for most
of the rest of this book, on that tiny
part of the electromagnetic spec
trum that can make the charges in
UV ----)10..,11-0..1--- VISIBLE - - - - - - - - - - - - -
=
1
I
1
I
3 X 10 14 Hz
7 X 10 14 Hz
Wavelength A =
400
500
V
Color =
the receptors of our eyes respond:
the visible. Figure 1.25 summarizes
some of the important things that
go on in and near this region of fre
quencies. At the top of this figure
we indicate the visible region. Short
wavelengths (400 nm) look Violet
(though we often refer to the short
wavelength end of the spectrum as
blue-see Sec. 10.4A). As we in-
600
FIGURE 1.25
Visible light and its interaction with life.
The frequencies (and wavelengths) of
light are marked along the top.
IR
-------------i._
700 nm
BGYORI
I
1
225 nm - .._ - - - - c - - - - - M o s t of sun's radiation
I
I
320 nm
I
----10-11.. .
Absorbed by
ozone (03) in
upper atmosphere
---------------~Io
1100 nm
2300 nm
~reaches
of sun's energy that
strongly by .1..
Absorbed by H2 0,
10 1.. Diminished
earth is here - - - - - - ' M - in atmosphere
--1-011---- CO , ozone (03) in
1-----
H20
2
atmosphere
I
I
I~ All that's left
I
I
-------l
l a t 25 m under I
the sea
r---
I
:
1
All that's left at 100 m under the sea
I
1
320 nm
I
"Hard UV"
-------1
stopped by glass
I
1
I
... :\
Skin response (sun
Effectiveness for
killing a bacillus
-s-----,
1
1
1
Insect vision
burn)~':! V
•. . il.
~
I
:
,.,
~
Human day vision [Photopic]
"
04 \ :
\.
'
\ ".
Human night vision [Scotopic)
\.
I
I
I
I
1
300
400
500
I
I
I"
I
....1 - - - - -
600
700 nm
1400 nm
1900 nm
Frequencies involved in "dark" chemistry - - - _ 1 0
I
Frequencies involved in (Iight·activated)
photochemistry
1
1
I
I
I
I
I
1
,.
'/\~~ Absorption spectrum of chlorophyll
_=-~
__~=-=-"",__'.::'"-'-_ Absorption spectrum of phytochrome
1
~
I
1
3200nm
:
Frequencies that excite phototropic behavior in plants
CHAPTER 1: FUNDAMENTAL PROPERTIES OF LIGlIT
24
crease the wavelength, the color
changes to Blue, then Green, Yel
low. Orange. and finally Red when
the wavelength gets to the long
wavelength end of the visible (700
run).
Most of the sun's radiation lies
between 225 nm and 3200 nm (see
also Fig. 1. 19), however. not all of
this penetrates the atmosphere and
reaches the earth's surface. -The
short wavelengths (below 320 nm)
are absorbed by the resonances of
ozone in the atmosphere. the long
wavelengths by water (above 1100
nm) and by carbon dioxide and
ozone (above 2300 nm). The result
is that about half of the sun's radia
tion reaching the earth lies in the
visible.
In the center of Figure 1.25 we
have plotted curves to indicate how
various systems respond to light of
different wavelengths. Thus. hu
man daytime vision is most effec
tive at about 555 run (yellow-green).
Human night vision uses different
receptors, which have their maxi
mum sensitivity toward the blue.
Actually, under very intense sources,
so intense that it feels warm, we
can see in the infrared OR) up to as
high as 1100 nm. Further. we could
see in the ultraviolet (UV) except
that the eye's lens absorbs UV. Peo
ple who have their lenses removed
(for cataracts) are sensitive doWn to
about 300' nm. Insects, however.
are most sensitive to ultraviolet
light. Since insects have little or no
vision in the red and yellow. we can
use yellow lights as "bug lights."
The yellow light provides useful il
lumination for our eyes, but the in
sects don't see it and therefore
aren't attracted to it. Conversely, to
attract bugs and zap them with
high voltage. we use blue or UV
bulbs.
Since most vertebrates have vi
sion in the same range as humans,
insects can have an interesting
kind of protective coloring. In the
visible, their coloring can be the
same as that of a poisonous insect
so that birds will leave them alone.
but their UV coloring can differ
from that of the poisonous insect so
that no mistakes are made when
they are looking for a mate.
Notice that your skin is most sen
sitive to UV of about 300 run wave
length. but that glass absorbs the
UV below 320 run-we don't sun
burn through a window. Notice also
that hard UV. below 300 run, kills
bacilli. but that the ozone in the
upper atmosphere absorbs this
short wavelength UV. thus saving
both bacilli and our skin from seri
ous damage.
In the range 250-1400 nm we
find not only vision, but all the
other light-dependent processes
critical to life. because the frequen
cies of the resonances of chemical
bonds occur in this range. Thus. at
the bottom of Figure 1.25 we show
the absorption spectrum of chloro
phyll (actually of several different
kinds combined). Since only the
green part of the spectrum is not
absorbed. it is reflected or transmit
ted. so chlorophyll looks green. The
chlorophyll responsible for the pho
totropiC· behavior of plants absorbs
only in the blue. Therefore, if we
grow plants in red light only, with
out blue light, the other chloro
phylls wlll absorb light and the
plants will grow, but not toward the
light. Also at the bottom of Figure
1.25 is the absorption spectrum of
phytochrome-the enzyme that pro
vides the "clock" for plants, deter
mining when they germinate. grow.
flower. and fruit. according to the
length of the night. It measures the
length of day by the amount of light
it absorbs in the red. (See the FO
CUS ON Light, Life, and the Atmo
sphere after this chapter.)
Light from sources other than the
sun may play a critical role in life.
Many organisms, such as the firefly,
are bioluminescent-emitttng their
own light as part of their mating
rites. (You can elicit a sexual re
sponse from a firefly with a flash
light, if you use the correct timing.)
Humans, as you know. also find
certain types of lighting to be ro
mantic. Probably the most extreme
effect of light on human sex life was
proposed by Shakespeare. Hamlet.
while feigning madness. suggests
that it is possible to get pregnant
from walking in the sun:
Hamlet:-Have you a daughter? Polonius: I have my lord. Hamlet: Let her not walk l' the sun: conception is a blessing; but as your
daughter may conceive,-frtend, look
to't.
Light certainly seems to playa role
in almost everything!
SUMMARY
-Greek tropos, turn. Therefore
phototropic means turning toward the
To see the light, it must pass from
a source, to an object (possibly).
and then to a detector. Light trav
els at about 300.000 km/sec (in vac
uum) and carries energy and m0
mentum. It is a type of wave
(propagating disturbance), but is
unusual in that it can propagate
through a vacuum-it is an elec
tromagnetic wave. An oscillating
charge creates a disturbance in the
electric fteld (which descrihes the
force on charged particles) as well
as in the magnetic fteld. Electro
magnetic waves of different fre
quencies are emitted and absorbed
by resonant systems (whose re
sponse is greatest at the resonance
.frequency).
Periodic waves are characterized
by frequency (v. number of oscilla
tions per second), wavelength (A,
separation
between
repeating
parts). amplitude (size or amount
of oscillation). pol.arl.zation (direc
tion of oscillation), and th,e direc
tion oj propagation (indicated by a
ray). The attrtbutes of light corre
spond to our sensations of color
(frequency, period, wavelength).
brightness (amplitude), and the di
rection from which the light ap
pears to come.
Visible light corresponds to a
very small range (wavelength 400 to
700 run) in the electromagnetic
spectrum, which ranges from the
very short wavelength gamma rays
and x-rays, through the ultraviolet
(UV). the viSible. the Uifrared (IR).
to the very long wavelength radio
light.
waves.
PROBLEMS
25 Black bodies emit a characteris
tic black-body spectrum in which
more energy is emitted from hot ob
jects than from cooler ones. Also,
the peak emission occurs at higher
frequencies for hot objects, lower
ing in the UV, and encased in a
frequencies for cooler objects. In
tube With a phosphorescent coat
ing, which converts the UV to visi
candescent electric lights consist
ble light.
of hot, gIowtngjilam.ents, whlleftu
orescent lights consist of gas glow
PROBLEMS
PI P2 P3 P4 P5 P6 P7 P8 (a)
(b)
Briefly Iist some of the properties of
light you have learned so far (e.g.,
how it travels, its speed, etc.).
When you look at the daytime sky,
away from the sun, it looks blue.
Explain where this light comes from
(Le., what is its source and how does
it get to your eyes?).
How do we know that light travels
through a vacuum?
Brand new windowpanes and mirrors
often come with pieces of tape on
them. Why?
(a) Which of the following are selfluminous objects (that is, we see
them by their own light, rather than
light reflected from them)? The sun,
the moon, a cat's eye, a television
picture, a photograph. (b) In the case
of the examples that are not selfluminous, what is the source of light
that allows you to see them?
Give an example of a resonance. In
your example, who (or what)
supplies the energy? (Example: A
parent pushing a child on a swing.
The parent supplies the energy.)
At Cornell, there used to be a narrow
suspension footbridge. If you walked
across it on a windless day, it
scarcely swayed at all. However, if
you jogged across it at just the right
speed, you could get it swaying
wildly. (That's why it's not there any
more!) Explain why there should be
this difference.
On November 7, 1940 at 10 A.M., a
47-mph wind set the Tacoma
Narrows Bridge into (torsional
twisting) vibration. The length of the
bridge's main span was about 850
meters. The wavelength of the waves
set up in the bridge was also 850
meters. Which of the two pictures in
the figure represents more nearly the
shape of the bridge that a snapshot
taken at the time would show?
P9 Which of the following common
everyday periodic phenomena are
examples of resonance? (a) A cork
bobbing up and down in waves in
the water. (b) Grandparent rocking in
his or her favorite rocking chair. (c) A
singer hitting a high note and
shattering a glass. (d) The people at a
rock concert stomping their feet in
rhythm with the music. (e) Floors of
the auditorium vibrating and cracking
as a result of the audience stomping
at the rock concert. (f) A rattle in
your car while idling, which stops as
the motor increases speed.
PI0 Your car is stuck in the snow, and
you can't push it hard enough to get
it over a hump of ice. It sometimes
hel ps to rock the car back and forth.
Apply some of our discussion of
vibrating systems to explain why this
helps.
P11 The figure below shows a picture of
a wave. Use a ruler and measure its
wavelength in centimeters.
P12 Redraw the wave of problem P11,
and label it "old wave." On top of it,
draw a wave of half the wavelength
and twice the amplitude, and label it
" new wave."
P13
Redraw the wave of Problem P11,
and label it "old wave." On top of it,
draw a wave of half the frequency
and the same amplitude, and label it
new wave."
P14 A light wave traveling in the vacuum
Ii
PIS P16 P17 P18 P19 P20 comes to a plate of glass. On
entering the glass, which, if any, of
the following increase, decrease,
remain unchanged: the frequency of
the wave, its wavelength, its speed?
What is the frequency of your
heartbeat while you are resting? Give
units with your answer, and tell how
you measured it.
light is an electromagnetic wave.
Does that mean that there must be
electrons present in the wave for it to
propagate? Explain.
Consider a radio wave and a visible
light wave. Which has a higher
frequency? Longer wavelength?
Longer period? Higher speed in
vacuum?
(a) The color of light corresponds (in
general) to which of the following
(there may be more than one
answer): frequency, speed,
wavelength, intensity, polarization?
(b) The brightness of light
corresponds (in general) to which of
the above list?
(a) As a black body becomes hotter,
does it emit more or less radiation?
(b) Does it radiate predominantly at a
higher or lower frequency?
Identify the type (e.g., UV, visible,
IR, etc.) of electromagnetic radiation
of each of the following wavelengths
in vacuum: 600 nm, 300 nm, 1400
nm, 21 cm, 0.1 nm, 3 km.
ClfAPTER 1: FVNDAMBNTAL PROPERTIES OF LIGHT
26
HARDER PROBLEMS
PHI How could you use a laser beam in a
dark room to determine the amount
of dust in the air?
PH2 What is the color of the sky on the
moon, where there is no atmosphere?
Why?
PH3 When soldiers march across a bridge,
they are told to break ranks. That is,
they are told not to walk in step with
each other. Think about the Walls of
Jericho and the Tacoma Narrows
Bridge, and explain why the soldiers
should break rank.
PH4 Cheap loudspeakers often have a
resonance in the sound frequency
region that we can hear (roughly SO
to 20,000 hertz). You get a much
larger output (sound) at the resonant
frequency, for a given input, than
you do at other frequencies. Better
speakers don't have such resonances.
Why is it bad to have such a
resonance? (Think about what you
would hear as someone played a
muscial scale.)
PHS (a) What is an electric field line?
(b) What do the arrows on an electric
field line convey? (c) How can one
physically determine whether an
electric field is present at a given
position?
PH6 The figure shows some wavefronts of
a light wave. Redraw the figure and
draw three different light rays on it.
PH7 Describe the physics of a standard
incandescent light bulb. (a) What
charges wiggle? What causes them to
wiggle? (b) Why is there a partial
vacuum-in the bulb? What gas is
present inside? Why? (c) Why don't
manufacturers simply make bulbs
that last forever? (Sure, they have to
stay in business, but what physical
constraint mitigates against making a
bulb that will last forever?)
PH8 Why can't we see x-rays directly?
("Because our eyes aren't sensitive to
x-rays" is correct, but not a suitable
answer. Why aren't our eyes
sensitive to x-rays?)
PH9 Objects A and B are illuminated by a
light source that produces only
visible radiation. To the eye, A looks
bright while B looks dark. Under the
same illumination, a photograph is
taken of the two objects, using film
sensitive only to infrared radiation. In
the picture, B shows up brighter than
A. Explain how this could be so.
lower than middle A? (As you go
down an octave, you divide the
frequency in half.) (c) Why do you
think organs have big pipes and
small pipes?
PM7 The figure shows (idealized) the way
the Tacoma Narrows Bridge vibrated
from 8 to 10 A.M. on November 7,
1940, shortly before it collapsed. The
length of the bridge's main span was
850 m. Each up and down oscillation
lasted 1f seconds. (a) This means that
the frequency of the oscillations was
v = 0.6 Hz. Show how one derives
this result from the data given above.
(b) What was the wavelength of the
waves set up in the bridge? Give the
reason for your answer. (c) What
was the speed of the waves? (Show
your calculation.)
MATHEMATICAL PROBLEMS
PMI How long does it take light to travel
from one of Galileo's hill tops to
another, 1.5 km away?
PM2 When a laser beam is sent to the
moon, reflected there, and returned
to earth, it takes 2.5 seconds for the
round trip. Calculate the distance to
the moon.
PM3 A rocket probe is sent to pass close
to the planet Jupiter. Suppose that at
the time the rocket reaches Jupiter,
Jupiter is 630,000,000 kilometers
from Earth. How long will it then
take a radio signal to travel from the
rocket to Earth?
PM4 A radio signal takes about 2.5 x
10- 3 seconds to travel from Boston
to Washington, D.C. Calculate the
distance between these two cities.
PMS (a) What is your height in meters?
(b) Express the result of part (a) in
millimeters and in nanometers.
(c) Which of these three units is more
reasonable to use for your height?
Explain your choice.
PM6 (a) The note that orchestras tune to,
middle A, has a frequency v = 440
Hz. The speed of sound in air is
about v = 330 m/s. What is the
wavelength of middle A? (b) What is
the wavelength of the A one octave
PM8 In Washington, D.C., radio station
WRC broadcasts on AM at 980 kHz.
Station WETA broadcasts on FM at
90.9 MHz. What are the wavelengths
(approximately) of the radio waves
used in each case? (Give the units
you use: e.g., meters, feet, cubits,
versts, etc.)
PM9 The range of wavelengths, in
vacuum, of visible light is about 400
nm to 700 nm. What range of
frequencies does that correspond to?
PMIO What is the frequency of 575 nm
(yellow) light?
PMII Consider a wave of wavelength 2 cm
and frequency 1 Hz. Cou.ld this be
an electromagnetic wave traveling in
vacuum? Why?
PMl2 If a black body is heated to a
temperature T (in degrees Kelvin,
K = ·C + 273), the most intense
radiation is at wavelength A (in
meters), where
A x T = 2.9
X
10- 3
(a) Find A for room temperature
bodies (take T = 290 K). (b) What is
the frequency of this radiation?
(c) What kind of radiation is this
(e.g., visible, IR, UV, x-ray, etc.)?
,
fOCUS ON . . .
Ught, life, and the atmosphere
That our atmosphere is so beneficent as
to transmit visible radiation from the
sun, but absorb killing ultraviolet, is a
consequence of its history. We will
outline its development so that we may
see the interplay of light, life, and the
atmosphere.
In the early days of the earth's
development, the atmosphere did not
contain many of the key ingredients of
life. There was no oxygen (0 2 ) and
consequently, no ozone (0 3 ), Nor was
there carbon dioxide (C0 2 ), As a result,
the ultraviolet (UV) radiation reached the
surface of the earth quite easily, much
more so than now (Fig. FO.1). This UV
provided just th,eright frequencies (i.e.,
it excited resomlflces) for various organic
molecules present in the seas to
combine into the first living organisms.
These primitive organisms had a better
chance for survival than they would have
now, since there were no other
organisms to eat them, and no oxygen to
oxidize them. Lacking oxygen, the
organisms could only get the energy they
needed to live by the process of
fermentation.· If you've seen beer
'Latin fermentare, to boil. This process
extracts energy by rearranging organic
molecules. For example:
ferment, you know that this process
produces carbon dioxide (C0 2 ), These
organisms did just that, pumping more
CO 2 into the atmosphere than currently
is there. The presence of the CO2 then
allowed new organisms to develop that
could live by photosynthesis.· These new
sugar -+ CO2
+
alcohol
+
energy
This process spends its "capital" (sugar) t9
'-produce energy, Once. the sugar is used up,
the process stops, It is a very inefficient
process. Only 5% of the chemical binding
energy in the sugar is released, and half of
that is lost as heat. (Fermentation tends to
heat up the surroundings, as anyone who
has made yeast bread will have noticed.)
The half not lost is stored in the molecule
ATP (adenosine triphosphate, the biological
"energy currency"), which then, if there is
raw material available, offers the energy for
new cell production.
·Greek synthesis, putting together, hence
putting together by Iight. Photosynthesis is
the process:
CO 2 + H 20 + light -+ sugar + O 2
It thus is a new source of sugar from which
organisms can derive energy, As such, this
is a crucial step in the evolution of life
because it allows organisms to take energy
from the sun continuously. That is, at this
point life developed a (very efficient) solar
battery.
FIGURE FO.t
r---------
Interaction of light and life.
I
I
I
I
~OG~s~
I
I
I
I
I
I
I
IL __ _
t
i
1
Cellular respiration
organisms removed some of the CO2 ,
fixing it in organic forms, and at the
same time produced molecular oxygen
(02 ), which entered the atmosphere,
with two critical effects. One was that
the sun's radiation converted some of
this O 2 into 0 3 (ozone). The ozone, as
we've seen (Fig. 1.25), blocked the
antibiotic UV from the earth's surface,
thus permitting living organisms to leave
the water for the land (about half a
billion years ago). This would not have
helped much unless there was a way the
organisms could produce energy more
efficiently. The process of fermentation
produced barely enough energy for
survival; none was left for motion. But
the second effect of the O 2 in the
atmosphere was to allow the much more
efficient process of cellula, respi,ation.*
This process uses the O 2 and replenishes
the CO 2 • Eventually these two balancing
processes, photosynthesis and cellular
respiration, came into equilibrium,
keeping the atmosphere roughly in its
present form for ages.
Currently, however, atmospheric CO 2
is increasing, largely because of the
destruction of the forests and the
burning of fossil fuels, which have raised
the CO 2 content in the atmosphere by
about 15% since 1850 (over 5% since
1958). This is thought to be responsible
for the warming tr~nd in northern
latitudes, from 1900 to 1940, by the
greenhouse effed. The idea is that CO 2 ,
like glass, lets the visible light through.
The earth absorbs this, warms up, and
radiates in the IR. But CO 2 (again like
glass) doesn't let the IR through, so the
"Latin respirare, to breathe. This is the way
we get most of our energy. The process is:
sugar
+
O2
.....
CO 2
+ H 20 + energy
This is the same process as burning sugar
over a flame, but we can do it at body
temperature in a more controlled fashion. It
is far more efficient than fermentation,
capturing over 85% of the chemical binding
energy in the sugar. Further, the by-products
are not harmful, unlike fermentation, which
usually produces something poisonous
(alcohol or some acid). With all this extra
energy, organisms can now do more than
exist, they can develop locomotion and
eventually paint the Mona L.sa. Yeast, on
the other hand, living by fermentation, do
not lead very active lives.
28
energy stays inside the CO 2 (or glass)
barrier and the earth gets hotter.· But
the story is more complicated. Although
atmospheric CO2 continues to increase,
a cooling trend seemed to appear in
1950. This may be due to other forms of
pollution in the atmosphere that may
reflect the incoming visible sunlight (like
metal foil, instead of glass) so that less
energy reaches the earth in the first
place. That is, there are many other
important molecules in our atmosphere
besides CO2 , such as nitrous oxide,
methane, ammonia, sulfur dioxide, and
even trace constituents. An important
question is whether they reflect the
sunlight (preventing the energy from
reaching the earth's surface), or transmit
the sunlight (allowing the energy
through), or absorb the sunlight (thus
heating up the atmosphere). The
greenhouse effect shows the
complication of transmission at one
frequency but not at another.
Another 'critical constituent is the
ozone, which protects us from the hard,
killing UV. It is unclear how sensitive the
ozone concentration is to various man
made effects such as supersonic
transports, fluorocarbons (commonly
used in aerosol spray cans), atmospheric
nuclear testing, or even agriculture (with
its effects on the nitrogen cycle).
However, a modest change in the ozone
content of the atmosphere can
significantly change the amount of hard
UV getting through, since most of it is
currently blocked. Too much of this UV
can cause mutants by altering the DNA
of which our genes are made, produce
skin cancer, and do other ecologically
more complicated things. Alternatively,
too little UV would prevent our bodies
from properly metabolizing calcium.
The effects of human activity on
atmospheric constituents, and their
effects in turn on climate and ecology
through the interplay of light with the
atmosphere, constitute one of the more
exciting fields of study today. The
consequences are, literally,
breathtaking.
'Actually, "greenhouse effect" is a bad
name. The major effect of an actual
greenhouse is to prevent the rising hot air
from escaping.
Principles of Geometrical Optics CHAPTER 2 2.1
-
INTRODUCTION
How do we so easily decide where to
place a beach umbrella to keep the
sun out of our eyes, without worry
ing about the electric field, the
wavefront, the wavelength, and the
frequency of the light? The answer
is that, for many simple problems it
is suffiCient to concentrate on the
light rays (Fig. 1. 17), the lines that
describe in a simple geometric way
the path of light propagation. Geo
metrical optics is the study of
those phenomena that can be un
derstood by a conSideration of the
light rays only. Geometrical optics
is useful as long as the objects with
which the light interacts are much
larger than the wavelength of the
light. As our beach umbrella is
about a million times larger than
the wavelength of visible light. geo
metrical optics is a very good ap
proximation for this and most other
everyday objects. For smaller objects
the beam will not propagate in only
one direction, but rather spread out
in all directions--much as sound
waves, with wavelength of about a
meter, spread out around obstacles
in the street.
In geometrical optics, then, light
(a)
does not bend around corners. We
think of the light as traveling in
straight lines as long as it is left
alone (Fig. 1.4). This straight-line
propagation enables us to locate the
beach umbrella so its shadow falls
on our eyes.
2.2
-
SHADOWS
T o cast shadows you need light
from a fairly concentrated source,
such as the sun. The best shadows
are cast by light that comes from
just one pOint: a point source,
which is an idealization, like a ray,
that can only be apprOximated, for
example, by a small light bulb or
candle. (Even a source as large as
the sun or a giant star can approx
imate a point source if it is far
enough away.) You also need a
screen, such as a flat, white sur
face, which redirects incident light
into all directions, so that you can
see the shadow (the light from the
surrounding area must enter your
eye).
If an obstacle blocks some of
the light rays headed for the screen,
the light rays that are not blocked
still reach the screen and make
that part of the screen bright.
Those that are blocked don't reach
the screen, so the places where
they would have hit are dark-a
shadow. We can figure out
the shadow's location by drawing
straight lines from the pOint source
to the edge of the obstacle and con
tinuing them to the screen. These
lines separate the regions where the
rays reach the screen from the re
gion where they are blocked. The
resulting shadow resembles the ob
stacle, but it is of course only a flat
(two-dimensional) representation of
the object's outline. Nonetheless, we
can easily recognize simple shapes,
such as a person's profile. Before
photography, it was popular to
trace people's shadows as silhouette
portraits (Fig. 2.1a). Today's x-ray
pictures are just shadows in x-ray
FIGURE 2.1
(a), (b) Various uses of shadows.
(c) Silhouette of one of the authors.
Etienne de Silhouette (1709-1767),
France's finance minister for a year, was
deposed because of his stinginess over
court salaries. He used cheap black
paper cutouts in place of conventional
decorations in his home and invented a
technique for making paper cutout
shadow portraits to raise money. When
he died, he was penniless and destitute,
a shadow of his former self.