* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nucleophilic Substitution Reactions
Survey
Document related concepts
Kinetic isotope effect wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Marcus theory wikipedia , lookup
Ene reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Asymmetric induction wikipedia , lookup
Transcript
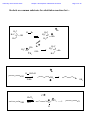
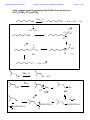
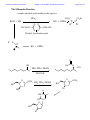
Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 1 of 20 Nucleophilic Substitution Reactions ¥ ¥ ¥ · There are several different kinds with different mechanisms They are polar and involve nucleophiles Substrates are usually alkyl halides, alcohols or esters of alcohols They can be used to prepare a wide variety of useful functional groups (p.267) Leaving Groups - good leaving groups are usually weak bases. d+ X C d- Br X C X C Br d+ d- X C OH HO O X C O O S CH3 X C O O S CH3 O Sulfonate Esters O C + Cl OH S O N R C O S O O O S O O CH3 O S O O O CF3 O > SO3 > Br > S O Order of "Leavibility" I R Cl > RCO2 CH3 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 2 of 20 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 3 of 20 Nucleophilicity - What makes a good nucleophile? - Basicity, polarizability or both Useful generalizations: 1) Bases are more nucleophilic than their conjugate acids. 2) When comparing the same atom type, the more basic are the better nucleophiles: 3) Going down a group of the periodic table, bigger atoms are better nucleophiles because their orbitals are more polarizable. Note: Iodide and bromide are good leaving groups and good nucleophiles. Two mechanisms apply to most substitution reactions: SN1 and SN2 SN1: Substitution, nucleophilic, unimolecular SN2: Substitution, nucleophilic, bimolecular Which mechanism applies depends on the structure of your substrate. Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 4 of 20 Classifying the Substrate CH3Br CH3CH2Br CH3CH2OH Cl H C H3C Cl Br OH H Br OH CH3 Cl OH H3C C H3C CH3 H3C H3C OH H OH Good substrates for SN1: Good substrates for SN2: Notice: Aryl and vinyl substrates do not undergo SN1 or SN2 reactions. Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 5 of 20 The SN1 Reaction 1) CH3 CH3 H3C C Br H3C CH3 2) + Br- C CH3 CH3 H3C CH3 :Nu C H3C CH3 C Nu CH3 The first step is the rate determining step. Note: Nucleophile concentration or nucleophile strength... Solvolysis reactions are SN1 reactions in which the solvent acts as the nucleophile. CH3 CH3 H2O/CH3CH2OH + ClH3C C H3C C Cl 1) CH3 CH3 CH3 H3C 2) CH3 H C CH3 H3C O H C H O CH3 H H H CH3 3) H3C C H O CH3 CH3 O H H H3C C O CH3 H O H H Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 6 of 20 SN1 reaction involve carbocation intermediates. So, the only substrates that undergo SN1 reactions are those that form stabilized carbocations. So - What makes a relatively stable carbocation? Br Br O Br Alkyl carbocations are stabilized by hyperconjugation. H H H H C H C H H C H H 1° Carbocation 3° Carbocation Vinyl and aryl carbocations... Cl C H H C C H C C H H C C H The order of stability for carbocations is... Cl Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 7 of 20 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 8 of 20 The Hammond Postulate - widely used to rationalize differences in reaction rate "The structure of a transition state resembles that species to which it is closest in energy." Early T.S. Late T.S. For endothermic reactions, the T.S. is closer in energy to products. The T.S. is said to be product-like in structure. It's also referred to as a "late" T.S. For exothermic reactions, the T.S. is closer in energy to reactants. The T.S. is said to be reactant-like in structure. It's also referred to as an "early" T.S. In the SN1 reaction, the rate determining step involving the formation of the carbocation intermediate is endothermic. The transition state CH3 CH3 Br CH3 H3C C CH3 + Br - + I - Energy +I - H3C C I H3C C CH3 CH3 + Br Br - +I - H3C C CH3 + Br - + I - Energy H3C C H H CH3 Reaction Coordinate Reaction Coordinate H H3C C I CH3 + Br - Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Reactions Involving Carbocations Substitution :Nu H3C C Nu C H3C H3C CH3 H3C CH3 Elimination H3C C H 3C CH2 H3C OH3 OH2 H C CH2 H 3C Addition H2C C H3C C H3C CH3 H H3C H3C C H CH3 H3C C H2 C CH 3 Rearrangement H3C H 3C C H Cl H3C H3C C AlCl3 C H2 Cl C H2 H Cl AlCl3 H3C H3C C CH3 H3C H3C C H3C H3C H3C C H3C Cl AlCl3 AlCl3 Cl AlCl3 Page 9 of 20 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 10 of 20 Rearrangments are often a problem when carbocation intermediates are present. CH3 H3C C H C H3C H 3C C H H and H C C H3C C CH(CH ) 3 C CH3 3 2 CH3 Cl 60% Cl 40% HCl -78° CH2 CH3 H C CH3 CH3 CH3 H2O, CH3OH, ∆ I CH3 H3C C HO H C H CH3 C H H C CH3 H C CH3 CH3 H3C C H CH3 CH3 C H H C HO CH3 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 11 of 20 Stereochemical Consequences of the SN1 Reaction Br C CH3 CH2CH3 Ph NaI/Acetone H 3C C CH2CH3 Ph S enantiomer I H3C C CH2CH3 Ph Sometimes, Racemization is not complete I I Br CH3 Br CH3 C C S CH2CH3 Ph H3C C CH2CH3 Ph R CH3 I C I CH2CH3 Ph Ph CH2CH3 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 12 of 20 The Mechanism of the Sn2 Reaction - one step With a charged nucleophile (i.e. Nu: = HO or NC ) and a leaving group, LG H dNu C d- LG HH + + Activated Complex H I H C H Br I C H H Br H H Nu:- C H LG H H Nu C H :LG- H The Stereochemical Consequences of the Sn2 Reaction NC- H3C CH3CH2 C Br H (S)-(+)-2-bromobutane dNC CH3 C CH3CH2 H + + dBr CH3 NC C CH2CH3 Br- H (R)-(-)-2-cyanobutane (In contrast, those reactions in which the stereocenter's configuration is unchanged are said to occur with retention of configuration.) Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 13 of 20 Why backside attack? Br C Br C C-Br s bonding orbital (from the overlap of sp3 orbitals on each) C-Br s* antibonding orbital Steric Effects in Sn2 Reactions - CH3 H 3C *Br CH3CH2 dd*Br C Br C Br H + + CH3 *Br CH3CH2 H H3C Methyl>1°>2°>>3° H3C Br Relative Rates 100 of reaction (label incorporation) C CH CH Br 2 3 H H3CH2C Br 1° Bromide 1.31 H3CH2CH2C Br 1° Bromide 0.81 C Br H H3C 2° Bromide 0.015 H3C C Br H 3C H3C 3° Bromide 0.004 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Solvent effects in substitution reactions O O Polar aprotic Polar protic C H 3C O H 2O CH3OH CH3CN H 3C CH3 H3CO OCH3 O O CH3CH2OH Page 14 of 20 S H CH3 C N(CH3)2 Polar, protic solvents facilitate SN1 reactions because they stabilize the transition state (which involves developing charges) ... d -- H Br H3C C H3C H H O H d++ H CH3 H O H Br H O H O H O H H 3C H H3C H O H Polar aprotic solvents facilitate SN2 because they destabilize the reactants (usually the charged nucleophile)... - H3C NC CH3CH2 C Br H d- NC CH3 C + + d- Br CH3CH2 H The transition state involves a diminishment of charge in the SN2 reaction. The Finkelstein always use the same solvent... Cl C H H O H H O H O + NaI Cl + NaCl H O H CH3 O H Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions The Effects of b-Branching in SN2 Reactions H H ddCH3CH2O C Br CH3CH2O H C Br H H H H3C Br Relative Rates of reaction 34.3 H3CH2C Br b a 1.95 + + H Br- CH3CH2O C H H H3CH2CH2C Br b Page 15 of 20 a H3CH2CH2CH2C Br H3CH2CH2CH2CH2C Br 0.60 0.44 CH3 b 0.41 CH3 a H3C C C Br H H2 0.058 b a H3C C C Br H2 CH3 0.0000083 Some SN2 reactions are complicated by the fact that the product may itself act as a nucleophile... H3N: Br Br NH2 NH3 Br + NH3 + NH4+ N H2 NaOH 16 eq. H3N: Br NH2 34% N H 57% N(CH2CH3)3 1% N H Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 16 of 20 Alcohols are common substrates for substitution reactions but ... H3C H3C C H Br H 3C H3C C Br OH H3C OH2 H3C Br CH3 H3C C CH3 H 3C H3C C OH2 H3C Br H OSO3H OH OH2 NaBr ZnCl2 OH HCl Br Br OH2 Cl O H ZnCl2 Cl Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 17 of 20 Other reagents useful in preparing alkyl halides from alcohols are SOCl2, SOBr2, PCl5 and PBr3 SOCl2, ∆ OH Cl O S Cl + SO2 + HCl Cl Cl O H OH Cl O O S O H Cl HCl Cl Cl O S O S 78% Cl Cl + SO2 + Cl PBr3, ∆ Br OH 60% Br PBr2 OH O H PBr2 Br O PBr2 HBr Br H Br O P[OCH(CH3)2]2 H O P[OCH(CH3)2]2 H O Br P[OCH(CH3)2]2 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 18 of 20 The Mitsonobu Reaction ...couples alcohols with weakly acidic species. CO2Et EtO2C :PPh3 RX + OPPh3 ROH + HX O O H3CH2CO N N H H OCH2CH3 N N Diethyl Azodicarboxylate X- R O RX + OPPh3 PPh3 O OH Ph O O OCH3 HN3, PPh3, DEAD 65% PhCO2H O HO OCH3 HN , PPh , DEAD 3 3 HO O HN O N O HO N3 HO N3 Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 19 of 20 Ethers... are usually formed by an SN2 process - the "Williamson Ether Synthesis" NaH, DMF OH Br O O Ethers can be cleaved by treatment with H-I or TMSI: (CH3)3SiÑI 2 HI O H O OH CH3 I 2 Br H O OH Br CH3 + H 2O H O CH3Br OH Chemistry 2500 Lecture Notes Chapter 7 Nucleophilic Substitution Reactions Page 20 of 20 Epoxides are cyclic ethers... The OÑ is a good leaving group due to... O :Nu N3 NaN3 O O EtOH, H2O OH Nu The nucleophile must attack the face opposite the O atom. Inversion is the rule. Under anionic conditions (Nu: ) the reaction is SN2 O O H H 3C HO H3C SPh H H3C SPh SPh Under acidic conditions the reaction is SN1-like... H O H+ H O H H3C H H3C H3C O H Nu :Nu H HO Cl , H2O pH = 7 H3C H H3C 91 O 9 H H3C O H Cl H Cl Cl H H3C Cl , H2O pH = 3.8 O 68 HO H H3C 32 Cl