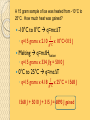* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Measurements - WordPress.com
Survey
Document related concepts
Passive solar building design wikipedia , lookup
Hypothermia wikipedia , lookup
Thermal conductivity wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Dynamic insulation wikipedia , lookup
Building insulation materials wikipedia , lookup
Underfloor heating wikipedia , lookup
Solar water heating wikipedia , lookup
Solar air conditioning wikipedia , lookup
Intercooler wikipedia , lookup
Heat exchanger wikipedia , lookup
Thermoregulation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
Heat equation wikipedia , lookup
Transcript
Unit 9 Thermochemistry Chapter 17 This tutorial is designed to help students understand scientific measurements. Objectives for this unit appear on the next slide. ◦ Each objective is linked to its description. ◦ Select the number at the front of the slide to go directly to its description. Throughout the tutorial, key words will be defined. ◦ Select the word to see its definition. Objectives 13 State the laws of thermodynamics 14 State the difference between temperature and heat 15 Identify variables and their labels for heat quantities and solve thermochemistry problems 16 Calculate heat-lost heat-gained quantities (calorimetry) 17 Interpret heating/cooling curves and identify the phase changes. 13 The Laws of Thermochemistry There are four laws of thermochemistry. The first three were written and then another was added. ◦ The one that was added was thought to be important enough that it should be listed first. Zeroth Law of Thermodynamics States that if two systems are at a thermal equilibrium with a third, they are at a thermal equilibrium with each other. ◦ Equilibrium occurs with there is no transfer of heat from one system to another. First Law of Thermodynamics States that energy can be transformed but it cannot be created or destroyed. ◦ This is sometimes known as the Law of Conservation of Energy. ◦ Since heat is a type of energy, this law applies to thermodynamics. Second Law of Thermodynamics States that heat always travels from hot to cold. ◦ Heat cannot be transferred from a colder object to a warmer object. Third Law of Thermodynamics States that absolute zero is the lowest temperature and all molecular motion stops at this point. ◦ Essentially, this means you cannot have a temperature lower than 0 K. Laws of Thermodynamics Recap 0th Law ◦ Thermal systems will form equilibrium relationships when mixed. 1st Law ◦ Energy cannot be created or destroyed. 2nd Law ◦ Heat flows from hot to cold. 3rd Law ◦ All molecular motion stops at absolute zero. 14 Temperature vs. Heat Temperature is often confused with heat but the two are quite different. Temperature is a measure of the average kinetic energy of molecules. Heat is the measure of the total kinetic energy of molecules. Temperature vs. Heat All molecules are in a state of motion. The motion is measured by kinetic energy. However, not all molecules are moving at the same speed and thus do not have the same kinetic energy. The average is taken to determine the speed of the majority of the molecules. The total is determined for a purpose that will be discussed in Unit 9. 15 Heat Quantities As discussed in Unit 10, temperature is the average kinetic energy of molecules. Heat is the total kinetic energy of molecules. Heat is measured in Joules or kiloJoules. To calculate heat, the following equation is used: q=mc∆T Heat Quantities q=mc∆T For the equation above: ◦ Q = heat measured in joules ◦ m = mass of the object in grams ◦ c = specific heat of an object in ◦ ∆T = change in temperature 𝐽 𝑔°𝐶 Specific Heat Every substance has a certain specific heat. This is the amount of heat required to raise the temperature of one gram of an object by one degree Celsius. Essentially, this number indicates how well on object holds heat. ◦ The higher the amount, the longer it will take to heat. Consider water. It has a high specific heat. Therefore, a lake can be cool even on a warm summer day. It takes the sun a long time to increase the temperature of the water. ◦ The lower the value, the shorter it will take to heat. Consider a sandy beach. It has a low specific heat. Therefore, it heats up fast on a warm summer day thus making it painful to walk on. Heat Quantities Since we have our equation, q=mc∆T, it is possible to calculate missing variables. Consider: These amounts are found on the back of the Periodic Table. A copper wire gained 570 J of heat when its temperature was increased from 25°C to 35°C. What is the mass? q = 570 J m = ? Grams c = 0.384 𝐽 𝑔°𝐶 q=mc∆T 570 J = X x 0.384 𝐽 𝑔°𝐶 x 10°C ∆T = 35°C - 25°C = 10°C x = 150 grams 16 Heat Lost/Heat Gained According to the laws of thermodynamics, when two systems are placed together they will form an equilibrium. Therefore, heat will travel from the warmer system to the colder system. Because energy cannot be created or destroyed, the heat lost by one system equals that gained by the other system. Calorimetry A common use for the heat lost/heat gained concept is calorimetry. Calorimetry is a technique used to determine information about an unknown object. It works by heating up an object and then placing it into water. The temperature of the water and the object will equilibrate to the same temperature. Calorimetry 1. Heat object 2. Place object in water 3. Determine temperature of system Calorimetry Consider: An sample metal with mass of 931 grams was heated to 45°C and placed in 200 grams of water. The water increased from 15°C to 25°C. What was the metal? Sample Water 931 200 0.449 4.18 Intial Temp. (°C) 45 15 Final Temp. (°C) 25 25 8360 8360 Mass (grams) Specific Heat (J/g°C) Heat (J) Iron Q=mc∆T These numbers will be the same for each column 17 Heating Curves As a substance gains heat, its molecules will speed up. Since molecules are speeding up, it is possible for a phase change to occur. During a phase change, the temperature does not increase even though the heat will. Melting Point = 20°C Boiling Point = 90°C Vaporization/Condensation Gas Temperature (°C) 90 Melting/Freezing Liquid 20 Solid Heat (J) Heating Curves and Heat Quantities A problem occurs when calculating a heat quantity when a phase change occurs. For instance, if ice is at 0°C and melts to a liquid, it gained heat. ◦ However, using q=mc∆T would not work because there was no ∆T. Heat of Fusion/Heat of Vaporization When a heat quantity needs to be calculated during a phase change, the equation is modified. Since each substance has a different specific heat for each phase and the temperature does not change, these variables are replaced. ◦ c∆T becomes ∆Hfusion for melting/freezing ◦ c∆T becomes ∆Hvaporization for condensation/vaporization q=mc∆T q=m∆H Heating Curves On the slants use q=mc∆T. On the flats use q=m∆H q=mc∆T q=m∆Hvaporization q=mc∆T q=m∆Hfusion q=mc∆T Heating Curves Consider: ◦ A 15 gram sample of ice was heated from -10°C to 25°C. How much heat was gained? This reaction undergoes a phase change at 0°C. A 15 gram sample of ice was heated from -10°C to 25°C. How much heat was gained? This this reaction touches three parts of the curve, it will take three equations to determine the heat gained. ◦ First, -10°C to 0°C q=mc∆T ◦ Second, melting q=m∆Hfusion ◦ Third, 0°C to 25°C q=mc∆T If these values are added together, we will have the total heat gained. A 15 gram sample of ice was heated from -10°C to 25°C. How much heat was gained? -10°C to 0°C q=mc∆T ◦ q=15 grams x 2.10 𝐽 𝑔°𝐶 x 10°C=315 J Melting q=m∆Hfusion ◦ q=15 grams x 334 J/g = 5010 J 0°C to 25°C q=mc∆T ◦ q=15 grams x 4.18 𝐽 𝑔°𝐶 x 25°C = 1568 J 1568 J + 5010 J + 315 J = 6893 J gained This concludes the tutorial on measurements. To try some practice problems, click here. To return to the objective page, click here. To exit the tutorial, hit escape. Definitions-Select the word to return to the tutorial