* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Half-Life - Chemistry 1 at NSBHS
Survey
Document related concepts
Diamond anvil cell wikipedia , lookup
Livermorium wikipedia , lookup
Chemical element wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Atomic theory wikipedia , lookup
Nuclear fission product wikipedia , lookup
Atomic nucleus wikipedia , lookup
Einsteinium wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Valley of stability wikipedia , lookup
Transcript
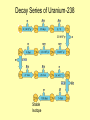
Rates of Nuclear Decay These stone tools from the archaeological site in Cactus Hill, Virginia, are at least 15,000 years old. Scientists estimated the age of the site based on rates of nuclear decay. Half-life Unlike chemical reaction rates, which vary with the conditions of a reaction, nuclear decay rates are constant. Half-life A half-life is the time required for one half of a sample of a radioisotope to decay. Half-life The half-life for the beta decay of iodine-131 is 8.07 days. Half-life The half-life for the beta decay of iodine-131 is 8.07 days. Half-life The half-life for the beta decay of iodine-131 is 8.07 days. Half-life The half-life for the beta decay of iodine-131 is 8.07 days. Half-life Every radioisotope decays at a specific rate. Half-lives can vary from fractions of a second to billions of years. Decay Series of Uranium-238 Stable Isotope Half-life Iridium-182 undergoes beta decay to form osmium-182. The half-life of iridium-182 is 15 minutes. a) After 15 minutes, how much iridium-182 will remain of an original 1 gram sample? 0.5 g b) After 30 minutes, how much iridium-182 will remain of the original 1 g sample? 0.25 g Assessment Questions 1. Cesium-137 has a half-life of 30 years. You find a sample with 3 g of cesium-137. How much cesium-137 existed in the sample 90 years ago? a. b. c. d. 9g 27 g 24 g 18 g Assessment Questions 1. Cesium-137 has a half-life of 30 years. You find a sample with 3 g of cesium-137. How much cesium-137 existed in the sample 90 years ago? a. b. c. d. 9g 27 g 24 g 18 g ANS: C Assessment Questions 2. What factors influence nuclear decay rates? a. b. c. d. pressure temperature concentration number of neutrons in nucleus Assessment Questions 2. What factors influence nuclear decay rates? a. b. c. d. pressure temperature concentration number of neutrons in nucleus ANS: D Transmutation Reactions • The conversion of an atom of one element to an atom of another element is called transmutation. Transmutation can occur by radioactive decay. Transmutation can also occur when particles bombard the nucleus of an atom. Transmutation Reactions • The first artificial transmutation reaction involved bombarding nitrogen gas with alpha particles. Transmutation Reactions • The elements in the periodic table with atomic numbers above 92, the atomic number of uranium, are called the transuranium elements. – All transuranium elements undergo transmutation. – None of the transuranium elements occur in nature, and all of them are radioactive. Transmutation Reactions • Transuranium elements are synthesized in nuclear reactors and nuclear accelerators.