* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Reactions
Survey
Document related concepts
Enantioselective synthesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Aromaticity wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydrogenation wikipedia , lookup
Marcus theory wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Ene reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Transcript
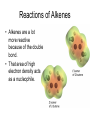
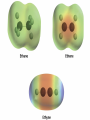
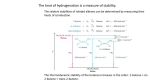
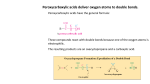
Organic Reactions Organic Reactions • Terms: – electrophile “love electrons” and – nucleophiles “love nuclei” • Reactions occur between electrophiles and nucleophiles. • Just about any part of a molecule can be either electrophilic or nucleophilic depending one what you are comparing it to Electrophiles “loves electrons” = attracted to negative charge Nucleophiles “loves nuclei” = attracted to positive charge maybe positively charged or often negatively charged or have deficit of electrons b/c atom lone pairs is attached to very electronegative high electronegativity atom carbon of carbonyl group acids alkenes Hydroxide –OH Chloride –Cl Ammonia – NH3 Kinds of Reactions • Just like in Chem 1 – there are classifications of organic reactions – Addition • like synthesis • Example: Electrophilic Addition – Elimination • kind of a mix between synthesis and decomposition – Substitution • like replacement reactions • Example: Nucleophilic Substitution Underlying principles of Organic • In order to predict reactions – and organic is all about predicting reactions so that you can control the product that’s formed • Identify the nucleophiles and electrophiles • Determine how electrons will move • Determine stability of reactants, products, and intermediates Reactions of Alkanes • Alkanes are relatively stable compared to other functional groups • Because there is no polarity in the molecule, no area of high electron density, the molecule doesn’t act as a nucleophile or an electrophile • Because of their stability, alkanes undergo a limited number of reactions: Reactions of Alkanes: Combustion • Complete: – • Reaction with enough oxygen to form CO2 and water Incomplete: – Reaction with not enough oxygen to make CO, C, and water • incomplete combustion = smoky, yellow flame – • CO is toxic for humans b/c it – • • The black soot is carbon and the yellow flame comes from glowing carbon atoms binds to hemoglobin in blood like oxygen and causes suffocation Unburned hydrocarbons react with other molecules in the air (like NO2) and make ozone which is also toxic for humans to breath. Particulates in the air from unburned hydrocarbons can also impair breathing ability. Reactions of Alkanes: Halogenation • Addition of Halogen – homolytic fission: molecule breaks evenly • Free Radical formed – Free radical is a molecule with an unpaired electron – Chlorination of methane H h H H+C lC l H m e th a n e H H h l H C C l C l H H ch lo ro m e th a n e h d ich lo ro m e th a n e H C l C l C l ch lo ro fo rm C l h C l C l C l te tra ch lo ro m e th a n e ls o :ca rb o n te tra ch lo rid e a ls o :trich lo ro m e th a n e a Steps in Free Radical Mechanism • Initiation: UV light separates Cl2 into free radicals • Propagation: more free radicals formed (2 steps) • Termination: two free radicals come together. UV Cl Cl Cl + CH4 CH3 + Cl Cl + (many ways for this to happen) Cl + Cl Cl Cl CH3 + CH3 H3C Cl H3C Cl + HCl Cl + Cl Cl Cl Reactions of Haloalkanes: Substitution • Similar to single replacement reactions, the halogen on an organic molecule is replaced with another halogen or an ion: H3C H3C Br Br - + Cl + - HO H3C H3C Cl OH + + - Br - Br Reactions of Alkenes • Alkenes are a lot more reactive because of the double bond. • That area of high electron density acts as a nucleophile. Rxns of Alkenes: Addition • Mechanism – Occurs through heterolytic fission • • formation of ion The first step is the attraction of the electrophile (Hydrogen ion) to the electrons in the pi bond. This forms a carbocation. The carbocation that is more substituted (has more carbons attached to it) is the most stable. The negatively charged halogen (nucleophile) adds to the carbocation H to form the halogenated alkene. • • H +H H H H CH 3 CH 3 C l + H + C H H Cl- CH 3 H H H C l Rxns of Alkenes: Addition • Hydrohalogenation – – – Alkene + acid halide monohaloalkane Halide ion adds to larger side (more substituted side of alkene) Secondary and tertiary carbocations are always more stable than a primary H H H CH3 + H Cl H Cl H H H CH3 Rxns of Alkenes: Addition • Hydration – – – – Alkene + water in acidic solution alcohol Acid acts as catalyst in rxn –OH group adds to larger side (more substituted side) of alkene Uses: hydration is used for commercial manufacture of ethanol H H H H H H acid + H O H H O H H H Rxns of Alkenes: Addition • Halogenation – – – – Alkene + halogen gas 1,2-dihaloalkane Diatomic gas has two atoms – both add to opposite sides of the double bond (and opposite sides of the molecule) Uses: Chlorine + ethane 1,2-dichloroethane: used as starting material for PVC Uses: Br2 dissolved in dichloromethane is used to distinguish between alkenes and alkanes. If reddish-brown color of Br2 disappears when added to unknown, the unknown has alkenes in it. MOVIE H H H H + Cl Cl Cl Cl H H H H Rxns of Alkenes: Addition • Hydrogenation – – – – – Alkene + Hydrogen gas (with catalyst) alkane Hydrogenation is saturating an unsaturated hydrocarbon Also called reduction (carbon is reduced in this reaction but is also reduced in many of the reactions above) Heterogeneous Catalyst: Pd or PtO2 (rxn occurs on a metal surface) Uses: unsaturated vegetable oils are saturated to produce saturated fats (more solid at room temp than unsaturated) for margarines H H PtO2/Pd H H + H H H H H H H H Addition Polymerization • Animation (Bag It) • Same Steps (Trash vortex) – Initiation (Free radical formed from UV) – Propagation • Initiator reacts with alkene to form a larger free radical • Propagation continues until it becomes more likely for free radical to find another free radical to react w/ rather than another alkene to react w/. – Termination • Two free radicals combine Trans isomer Cis isomer