* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Class 3 updated Sep 30 2011
Atomic theory wikipedia , lookup
Plasma polymerization wikipedia , lookup
History of molecular theory wikipedia , lookup
Metallic bonding wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Ceramic engineering wikipedia , lookup
Synthesis of carbon nanotubes wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Double layer forces wikipedia , lookup
Electrochemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Theory of solar cells wikipedia , lookup
Gaseous detection device wikipedia , lookup
Thermal spraying wikipedia , lookup
MEMS
Class 3
Fabrication Processes for MEMS
Mohammad Kilani
{111} planes has slow etch rate in KOH solution
perspective view of a {100} wafer and
a KOH-etched pit bounded by {111}
planes
ODE etching with concave corners in the mask openings
Square and rectangular openings in mask with the silicon etched for short or
long time. Anisotropic etching or Orientation Dependent Etching (ODE).
ODE etching with convex corners in the mask openings
Etching at convex corners and the formation of
suspended beams of a material that is not etched
(e.g., silicon nitride, p++ silicon). The {411} planes
are frequently the fastest etching and appear at
convex corners.
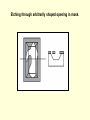
Etching through arbitrarily shaped opening in mask.
Wafer Processes
Silicon Production by Reduction
•
•
•
•
•
•
Silicon is commercially prepared by the heating of
high-purity silica in an electric arc furnace using carbon
electrodes. At temperatures over 1900 °C
Carbon reduces the silica to silicon according to the
chemical equation SiO2 + C → Si + CO2
Liquid silicon collects in the bottom of the furnace, and
is then drained and cooled
The silicon produced via this process is called
metallurgical grade silicon and is at least 99% pure.
Using this method, silicon carbide, SiC, can form.
However, provided the amount of SiO2 is kept high,
silicon carbide may be eliminated, as explained by this
equation: 2SiC + SiO2 → 3Si + 2CO
In 2000, metallurgical grade silicon cost about $ 0.56
per pound ($1.23/kg)
Silicon Purificattion
(Zone Melting Method)
The first silicon purification method to be
widely used industrially is the zone
method. Rods of metallurgical grade
silicon are heated to melt at one end.
Then, the heater is slowly moved down the
length of the rod, keeping a small length of
the rod molten as the silicon cools and
resolidifies behind it. Since most impurities
tend to remain in the molten region rather
than resolidify, when the process is
complete, most of the impurities in the rod
will have been moved into the end that
was the last to be melted. This end is then
cut off and discarded, and the process
repeated if a still higher purity was desired
Silicon Purificattion
(Siemens Process)
Today, silicon is purified by converting it to a silicon compound that
can be more easily purified than silicon itself, and then converting
that silicon compound back into pure silicon. Trichlorosilane is the
silicon compound most commonly used as the intermediate. In
the Siemens process, high-purity silicon rods are exposed to
trichlorosilane at 1150 °C. The trichlorosilane gas decomposes
and deposits additional silicon onto the rods, enlarging them
according to chemical reactions like
2 HSiCl3 → Si + 2 HCl + SiCl4
Silicon produced from this and similar processes is called
polycrystalline silicon. Polycrystalline silicon typically has impurity
levels of 1 part per billion or less.
Silicon Crystallization
Czochralski process
The majority of silicon crystals grown for device
production are produced by the Czochralski process,
since it is the cheapest method available. A small seed
crystal of the material to be grown is lowered to the
surface of the melt and then drawn upwards, slowly. As
the seed crystal is pulled from the melt, it draws with it a
layer of molten material. This material cools gradually,
taking on the same crystalline structure as the seed
crystal. Pellets of dopant material are added to the melt
if extrinsic semiconductor material is required. The
process is named after Jan Czochralski, who
discovered the method in 1916 while investigating the
crystallization rates of metals.
Silicon Crystallization
Float-Zone process
When silicon is grown by the Czochralski method the melt is contained in a silica
(quartz) crucible. During growth the walls of the crucible dissolve into the melt
and Czochralski silicon therefore contains oxygen impurities with a typical
concentration of 1018cm − 3. For certain electronic devices, particularly those
required for high power applications, silicon grown by the Czochralski method is
not pure enough. For these applications, float-zone silicon (FZ-Si) can be used
instead. Up to 99.99999999999% (11 nines) purity may be obtained
Wafer Preperation
•
•
•
•
•
•
•
Size the boule’s diameter by grinding on a lathe
X-ray orient Accuracy 0.5 deg. In the best case.
Grind one or more flat along the length of the boule
slice
Lapping with Al2O3 to and glycerine to produce a
uniform flatness to within 2 µm.
Wafer edge rounding to minimize the source of
cracks
Polishing and cleaning
Flat assignments
Flats at 180 deg for n-type and 90 deg for p-type
Flats at 45 deg for n-type, no secondary for p-type.
Basic Microfabrication Processes
Basic Processes in Micromachining
•
•
•
Deposition
– Oxidation
– Physical Deposition:
(deposition takes place
without the need for
chemical reaction) spin
coating, evaporation,
sputtering, ion
implantation.
– Chemical Deposition
(deposition results from a
chemical reaction)
LPCVD, APCVD, PECVD,
epitaxy,
Lithography
Removal
– Wet isotropic etching
– Wet anisotropic etching
– Plasma etching
– Reactive Ion Etching (RIE)
– Deep Reactive Ion Etching
(DRIE)
Deposition - Oxidation
•
•
•
•
•
Silicon dioxide (SiO2) is
thermally grown by oxidizing
silicon in oxygen (Dry oxidation)
or in steam (Wet oxidation).
Used as a common insulating
layer, as a mask, or as a
sacrificial material.
Growth occurs at 800-1200 C.
Wet dioxide: less dense that dry
oxide but faster growing.
0.5 µm (dry) → ~6 hours / 1 µm
(wet) → 1 hour
Si(solid) + O2(gas) → SiO2
Si(solid) + 2H2O (gas) → SiO2 + 2H2
(Dry)
(Wet)
Deposition - Oxidation
O2
Silicon
xox
SiO2
xs
Silicon
SixNy
SiO2
Silicon
•
After the first layer of SiO2is formed (~25 Å), diffusion is
heat driven
•
Silicon is consumed as the silicon dioxide is grown.
•
The ratio of silicon thickness converted xs to oxide
thickness formed xox is 0.46
•
~2m films are maximum practical
•
Oxidation can be masked with silicon nitride, which
prevents O2 diffusion
Deposition Issues- Conformality
A conformal coating covers all
surfaces to a uniform depth
A planarizing coating tends to
reduce the vertical step height
of the cross-section
A non-conformal coating deposits
more on top surfaces than bottom
and/or side surfaces (line of sight)
Physical Deposition-Spin Coating
•
•
•
•
•
Viscous liquid (usually photoresist) is poured on center of wafer
Wafer spins at 1000-5000 RPM for ~30s
Baked on hotplates 80-500C for 10-1000s
Application of etchants and solvents, rinsing
Deposition of polymers, sol-gel precursors
Physical Deposition - Evaporation
•
•
•
•
•
•
Based on the boiling off (or sublimating) of a
heated material onto a substrate.
Can be used to deposit single element
conductors, resistors (metalization e.g.
aluminum and gold) and dielectrics e.g SiO2.
Alloy depositions are difficult to control because
the evaporation rates of components are usually
different.
Very high-vacuum required to prevent oxidation
Typically line-of-sight deposition. Rotating
substrate holders (planetary holders) can be
used to achieve more uniform deposition and
step coverage.
Commonly used heating sources include
resistive heating, electron beam bombardment
and RF induction heating.
Evaporation – Resistive Heating
•
•
•
Obtained by passing a high current through a highly refractory metal
containment structure (e.g. a tungsten or tantalum filament).
May be used to evaporate aluminum and gold In a lab setting
Source contamination may occur as the source metal reacts with the
crucible at high temepratures
Evaporation – Electron Beam (e-beam)
•
•
•
•
•
•
Refractory metals (e.g. tungsten) and
dielectrics (e.g. SiO2) are evaporated by
e-beam bombardement.
A high intensity e-beam gun is focused
on the target meterial that is placed on
water cooled copper hearth.
E- beam is magnetically focused onto the
evaporant, which melts locally.
The evaporant metal forms its own
crucible and the contact with the hearth is
too cool for chemical reaction.
Results in fewer source contamination
problems than in the case of resistive
heating.
Disadvantage: The process may cause
x-ray damage or ion damage on the
substrate
DC Plasma
•
•
•
Low pressure
inert gas
CATHODE
(-)
+ +
•
A plasma is an electrically neutral, highly ionized gas composed of ions,
electrons, and neutral particles. It is a phase of matter distinct from solids,
liquids, and normal gases
Plasma may be obtained by applying a high voltage between two opposed
parallel plate electrodes in a low pressure (0.001 – 1 Torr) inert gas (argon)
chamber.
Applying a high voltage (1.5 kV) between the anode and the cathode produces
a 100 V/cm field in a 15 cm separted electrodes.
Electrical breakdowm of the argon gas occurs when an electron collides with
the argon atom with a kinetic energy sufficient to free an electron from the
atom, thus producing a second electron and a positive ion. (ionization
potential)
Both free electrons reenergize creating an avalanche of ions and electrons
that results in a gas breakdown emitting a characteristic glow. The voltage
between the electrodes drops from 1.5 kV to about 150 V.
- -
•
ANODE
(+)
DC Plasma
When ions strike a material surface, secondary electron (Auger
electrons) are generated, which sustain the plasma. Additionally
depending on the energy of the ions, the following may happen to
them:
–
–
–
–
Bouncing off the surface
Absorption by the surface to produce heat
Penetration of the surface to deposit the energy within the
material
Ejection of surface atoms from the cathode (sputtered)
Low pressure
inert gas
+ +
CATHODE
(-)
- -
•
ANODE
(+)
Deposition – Sputtering
•
•
•
•
•
Removes surface atoms or molecular fragments
from a solid cathode (target), by bombarding it
with positive ions from an inert gas (argon)
discharge, and deposit some of these atoms or
molecular fragments on the nearby substrate to
form a thin film.
Used for deposition of compound materials and
refractive metals that are difficult to evaporate.
Sputtered metals and dielectrics
– Argon plasma sputters material (small #s of
atoms) off target
– Ejected material takes ballistic path to
wafers
Typically line-of-sight from a distributed source
Requires high vacuum depending on material
RF Plasma
•
•
•
•
A radio frequency (RF) voltage is applied between the two electrodes causing
the free electrons to oscillate and to collide with gas molecules leading to a
sustainable plasma.
Ionization may be generated at a pressure lower than in DC plasma.
Allows working with dielectrics as well as metals.
The target substance is placed at the cathode
Low pressure
inert gas
CATHODE
(-)
ANODE
(+)
Deposition Issues - Compatibility
•
•
Thermal compatibility
– Thermal oxidation and LPCVD films are mutually compatible
– Thermal oxidation and LPCVD are not compatible with polymers
(melting/burning) and most metals (eutectic formation, diffusion, furnace
contamination)
Topographic compatibility
– Can not spin-cast over large step heights
– Distributed-source deposition over deep trenches leaves keyholes
Deposition - Chemical Vapor Deposition (CVD)
•
•
•
•
Can be used to deposit metals,
dielectrics, polysilicon and other
compounds.
The reactants for the desired film
are introduced into the CVD
chamber in their vapor phase.
Gases dissociate on surfaces at
high temperature. They
chemically react at the heated
surface of the wafer to form the
desired film.
Can take place at atmospheric
pressure (APCVD) or low pressure
(LPCVD). The later is the typical
approach.
Deposition - Chemical Vapor Deposition (CVD)
•
•
•
•
•
Offers conformal coating, with good step
coverage.
Can coat a large number of wafers at a time
(batch fabrication)
LPCVD pressures around 300mT (0.05%
atmosphere)
Moderate Temperatures
– 450 SiO2
– 580-650 polysilicon
– 800 SixNy
Very dangerous gases
– Silane: SiH4
– Arsine, phosphine, diborane: AsH3, PH3,
B2H6
Deposition - Epitaxy
•
•
•
•
A special type of CVD refering to growing a
single crystalline layer upon a single
crystalline substrate
Homoepitaxy is the growth of the same type
of material on the substrate (e.g., p+ silicon
etch stop layer on a n-type substrate for a
diaphragm formation)
Heteroepitaxy is the growth of one materials
on a substrate which is a different material
(e.g., GaAlAs on GaAs).
Silicon homoepitaxy is used in bulk
micromachining for the etch stop layers.
Chemical Vapor Deposition (CVD)
P4, 2.25 microns LPCVD
S4, 2.0 microns PECVD
Dimple 4 gap
0.2 microns
P3, 2.25 microns LPCVD
Dimple 3 gap
S3, 2.0 microns PECVD 0.4 microns
P2, 1.5 microns
S2, 0.3 microns
P1, 1.0 microns
Dimple 1 gap
S1, 2.0 microns LPCVD
0.5 microns
P0, 0.3 microns
Silicon Nitride, 0.8 microns
Thermal SiO2, 0.63 microns
Substrate
6-inch wafer, <100> n-type
Doping
•
•
•
Controlled quantities of impurity atoms are
introduced into selected regions of the
semiconductor through masks on the top of
the wafer.
Used to selectively dope the substrate to
produce either an n- or p- type region
Doped regions are used to fabricate passive
and active electronic components, as well as
etch stop layers and conductive polysilicon
micromechanical devices.
Diffusion Doping
•
•
•
Diffusion is done by placing wafers in a hightemperature furnace and passing a carrier
gas that contains the desired dopant through
the wafer.
For silicon, boron is the most popular p-type
dopant (acceptors), while arsenic and
phosphorous are n-type dopants (donors).
Silicon dioxide is usually used as the
diffusion mask for silicon wafers.
Diffusion Doping
•
•
•
•
There are two major steps in diffusion
doping: predeposition and drive-in.
During predeposition, impurity atoms are
transported from the source onto the wafer
surface and diffused into the wafer. The
number of atoms that enter the wafer
surface is limited by the solid solubility of the
dopant in the wafer.
The second step is drive-in, where the
deposited wafer is heated in a diffusion
furnace with an oxidizing or inert gas to
redistribute the dopant in the wafer to reach
a desired depth from the surface.
After deposition the wafers have a thin
highly-doped oxide layer (glaze) on the
silicon. A sub-step called deglaze is usually
used to remove this oxide layer by an HF dip
of the wafer. For p+ (a very heavily doped)
predeposited wafers, a low temperature
oxidation process may be used to facilitate
the removal of the "glaze" layer.
Ion Implantation
•
•
•
A technique for introducing impurity atoms
into a wafer below the surface by
bombarding it with a beam of energetic
impurity ions.
Offers precise control over the number and
location of the impurity atoms in the
substrate. It is used extensively in VLSI
fabrication.
The projected range, the projected straggle
and the distribution profile are dependent on
the energy of the ions and the properties of
the implant mask. The crystal damage and
the annealing process, as well as the
method used to avoid ion-channeling are
other considerations in this process.
Photolithography
Surface Cleaning
Removal of particulates, organic films, adsorbed metal ions
Adhesion Promoter
Sometimes used to achieve better adhesion of the resist
Resist Application
Thickness varies with rotational speed of the spinner and
viscosity of the resist
Prebake (Soft Bake)
70 °C – 90 ºC, necessary to drive solvent out of the resist
Exposure
Contact/proximity printing, projection printing
Developing
Negative resist: solvent; positive resist: alkaline developer
Post Bake
90 ºC – 140 ºC, necessary to increase both adherence
and etch resistance
Etching/deposition/doping
Resist Removal
Stripping solutions, plasma etching in oxygene atmosphere
Critical Cleaning
1
1 Organics
H2SO4 +
H2O2
H2O Rinse
2
2 Oxides
HF +
H2O
H2O Rinse
3
4
3 Particles
4 Metals
NH4OH +
HCl +
H2O2 + H2O
H2O2 + H2O
H2O Rinse
H2O Rinse
5
5 Dry
H2O or IPA +
N2