* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 mole
Survey
Document related concepts
Chemical element wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Isotopic labeling wikipedia , lookup
Mass spectrometry wikipedia , lookup
History of molecular theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Transcript
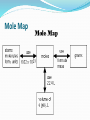
11.2 Mass and the Mole Objectives: Calculate the number of moles in a given mass of an element and the mass of a given number of moles of an element Calculate the number of moles of an element when given the number of atoms of the element Calculate the number of atoms of an element when given the number of moles of the element 11.2 Mass and the Mole Vocabulary Molar mass The Mass of a Mole Moles of different substances have different masses just like a dozen tennis balls has a different mass than a dozen baseballs The Mass of a Mole Molar Mass- The mass in grams of one mole of any pure substance Units: grams/mol Example: Manganese has an atomic mass of 54.94 amu there fore it has a molar mass of 54.94 g/mol PROBLEM: FIND THE MOLAR MASS OF: 1) Mn 54.9 g/mol 2) Ca 40.1 g/mol 3) Xe 131.3 g/mol 4) How many particles do you have of each? 5) If you had 6.02 x 1023 atoms of uranium, how many grams would you have? •When you measure the molar mass of an element on the balance, you are measuring 6.02 x 1023 atoms! Using molar mass # of moles X # of grams = mass 1 mole # grams X 1 mole = # of grams moles Using molar mass Converting mass to moles and moles to mass Given: Given: Multiply by M.M Divide by M.M Mole Map PROBLEMS: 1) How many grams in 3.00 mol C? Note: molar mass of C: 1 mole = 12.0 g 3.00 mol C x __12.0 g 1 mole = 36.0 g C 2) Calculate the moles in 3.2 g Oxygen, O2. Molar mass: 1 mol=32.0g 3.2 g O x 1 mole 32.0 g = 0.10 mol O2 PROBLEM - Mass to Atoms Conversions (2 steps) Find the atoms in 25.0 g of Gold (Au). Step 1: Find moles Au (use molar mass) 25.0 g Au x 1 mole 197.0 g = 0.1269 mol (keep digits in calc.) Step 2: Find atoms (Av.#) 0.1269 mol x 6.02 x 1023 atom 1 mole = 7.64 x 1022 atoms Au PROBLEM: Calculate the mass of 4.0 x 1022 atoms Al. Use mole map to draw out path from atoms g Step 1: Use Avogadro’s # to find # moles: 4.0 x 1022 atoms Al x ___1 mole 6.02 x 1023 atoms = 0.66 mol Al Step 2: Use molar mass to convert to grams: 0.664 mol Al x 27.0 g 1 mol = 1.8 g (round off at end!) http://www.moleday.org/htdocs/gifs/Moles%20of%20the%20Caribbean[1].JPG 11.3 Moles of Compounds Objectives: Recognize the mole relationships shown by a chemical formula Calculate the molar mass of a compound Calculate the number of moles or a compound and the mass of a compound from a given number of moles of compound Determine the number of atoms or ions in a mass of a compound Chemical Formulas and the Mole Ratio The subscripts within a chemical formula also represent molar ratios among the elements of the compound Consider the compound: CCl2F2 The ratio of elements is: C to Cl to F 1 : 2 : 2 Thus there is a molar ratio of 1 mole of carbon to 2 moles of chlorine to 2 moles of fluorine. Chemical Formulas and the Mole Ratio The compound to element mole ratio is: Consider the compound: 1 mole of CCl2F2 The ratio of elements is: 1 mol C to 1 mole of CCl2F2 2 mol Cl to 1 mole of CCl2F2 2 mol F to 1 mole of CCl2F2 1:1 2:1 2:1 The Molar Mass of Compounds Molar mass of a compound is determined by adding the masses of all elements present. # of moles x molar mass = number of grams Example: K2CrO4 2 mol K x 39.10 g K = 78.20 g 1 mol K 1 mol Cr x 52.00 g Cr = 52.00 g 1 mol Cr 4 mol O x 16.00 g O = 64.00 g 1 mol O 78.20 g 52.00 g + 64.00 g 194.20 g K2CrO4 CHEMICAL FORMULA Converting to moles of individual atoms (or ions) given formula: C6H12O6 (glucose): 1.0 mole glucose contains: 6.0 mol C atoms 12. mol H atoms 6.0 mol O atoms Problem: How many moles of each atom in Phosphoric acid, H3PO4 3 mol H atoms, 1 mol P at, 4 mol O at. MOLAR MASS OF COMPOUNDS: 1) Find molar mass of water, H2O. # moles X molar mass = # grams (formula) (periodic table) H= 2 O=1 Total x 1.0 g x 16.0g = 2.0 g =16.0 g 18.0 g = 1 mole Converting moles to mass: PROBLEM: Find mass of 3.2 moles ammonia, NH3. Find molar mass of NH3 & plug in: N = 1 x 14.0 g = 14.0 g H = 3 x 1.0 g = 3.0 g 17.0 g = 1 mol 3.2 mol NH3 x __17.0 g_____ 1 mol = 54 g NH3. = Converting mass to moles: PROBLEM: Find moles in 8.8 g of CO2 . 8.8 g CO2 x 1 mol g Find molar mass CO2: C = 1 x 12.0 = 12.0 O = 2 x 16.0 = 32.0 44.0g/mol 8.8 g CO2 x 1 mol 44.0 g = 0.20 mol CO2.