* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 7_honors
Survey
Document related concepts
Bremsstrahlung wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Rigid rotor wikipedia , lookup
History of molecular theory wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Magnetorotational instability wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Metalloprotein wikipedia , lookup
Molecular dynamics wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Atomic theory wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Transcript
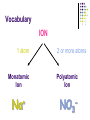
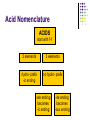
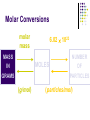
Chapter 7 Chemical Formulas & Chemical Compounds Vocabulary CHEMICAL FORMULA IONIC COVALENT Formula Unit Molecular Formula NaCl CO2 Vocabulary COMPOUND 2 elements Binary Compound NaCl more than 2 elements Ternary Compound NaNO3 Vocabulary ION 1 atom 2 or more atoms Monatomic Ion Polyatomic Ion + Na NO3 - Ionic Nomenclature Ionic Formulas Write each ion, cation first. Don’t show charges in the final formula. Overall charge must equal zero. If charges cancel, just write symbols. If not, use subscripts to balance charges. Use parentheses to show more than one polyatomic ion. Stock System - Roman numerals indicate the ion’s charge. Ionic Nomenclature Ionic Names Write the names of both ions, cation first. Change ending of monatomic ions to -ide. Polyatomic ions have special names. Stock System - Use Roman numerals to show the ion’s charge if more than one is possible. Overall charge must equal zero. Ionic Nomenclature Consider the following: Does it contain a polyatomic ion? 2 elements no -ate, -ite, 3+ elements yes -ide, Does it contain a Roman numeral? Check No the table for metals not in Groups 1 or 2. prefixes! Ionic Nomenclature Common Ion Charges 1+ 0 2+ 3+ NA 3- 2- 1- Ionic Nomenclature potassium chloride K+ Cl- KCl Mg(NO3)2 CuCl2 magnesium nitrate Mg2+ NO3- copper(II) chloride Cu2+ Cl- Ionic Nomenclature NaBr sodium Na2CO3 sodium bromide carbonate FeCl3 iron(III) chloride Oxidation Numbers Oxidation Numbers (aka Oxidation States) are assigned to atoms composing a compound or ion in order to indicate the general distribution of electrons among the bonded atoms. Oxidation Numbers are very helpful in naming compounds, writing formulas & in balancing chemical equations. Assigning Oxidation Numbers 1. The atoms in a pure element have an ox # equal to ZERO. (ex. Na, O2, P4, S8) 2. The more electronegative element in a binary molecular compound is assigned the # equal to the negative charge it would have as an anion. Likewise the less electronegative element is assigned the positive charge as the cation. Assigning Oxidation Numbers (con’t) 3. Fluorine has an oxidation number of -1 in all of its compounds. Why? 4. Oxygen has an oxidation number of -2 in most compounds. Exceptions include the peroxides (such as H2O2 => ox# = -1) and when combined with halogens (ex. OF2=> ox# = +2) Assigning Oxidation Numbers (con’t) 5. Hydrogen has an ox# = +1 in all compounds containing elements that are more electronegative than it. However it has an ox# = -1 in compounds with metals. 6. The algebraic sum of the oxidation numbers of all atoms in a neutral compound is equal to zero. Assigning Oxidation Numbers (con’t) 7. The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion. 8. Although rules 1-7 apply to molecular compounds, ox #’s can be assigned to atoms in ionic compounds as well. Practice: Assigning Oxidation Numbers Try these now: HCl CF4 PCl3 SO2 HNO3 KH P4O10 Practice: Assigning Oxidation Numbers Try these now: HClO3 N2O5 MgCl2 GeCl2 H2SO4 Mo2O3 Molecular Nomenclature Prefix System (binary compounds) 1. Less e-neg atom comes first. 2. Add prefixes to indicate # of atoms. Omit monoprefix on first element. 3. Change the ending of the second element to -ide. Molecular Nomenclature PREFIX monoditritetrapentahexaheptaoctanonadeca- NUMBER 1 2 3 4 5 6 7 8 9 10 Molecular Nomenclature CCl4 carbon tetrachloride N 2O dinitrogen monoxide SF6 sulfur hexafluoride Molecular Nomenclature arsenic trichloride AsCl3 dinitrogen pentoxide N2O5 tetraphosphorus decoxide P4O10 Molecular Nomenclature The Seven Diatomic Elements Br2 I2 N2 Cl2 H2 O2 F2 H N O F Cl Br I Definition Acids Compounds Formulas that form H+ in water. usually begin with ‘H’. Examples: HCl – hydrochloric acid HNO3 – nitric acid H2SO4 – sulfuric acid Acid Nomenclature Anion Ending Acid Name -ide hydro-(stem)-ic acid -ate (stem)-ic acid -ite (stem)-ous acid Acid Nomenclature ACIDS start with 'H' 2 elements 3 elements hydro- prefix -ic ending no hydro- prefix -ate ending becomes -ic ending -ite ending becomes -ous ending Acid Nomenclature HBr 2 hydrobromic acid carbonic acid sulfurous acid H2CO3 3 elements, -ide elements, -ate H2SO3 3 elements, -ite Acid Nomenclature hydrofluoric acid 2 H+ F- HF H+ SO42- H2SO4 H+ NO2- HNO2 sulfuric acid 3 elements elements, -ic nitrous acid 3 elements, -ous Molar Conversions Review of the Mole What is the Mole? A counting number (like a dozen) Avogadro’s number (NA) 1 mol = 6.02 1023 items A large amount!!!! What is the Mole? 1 mole of hockey pucks would equal the mass of the moon! 1 mole of basketballs would fill a bag the size of the earth! 1 mole of pennies would cover the Earth 1/4 mile deep! Molar Mass Mass of 1 mole of an element or compound. Atomic mass tells the... atomic mass units per atom (amu) grams per mole (g/mol) Molar Mass Examples carbon 12.01 g/mol aluminum 26.98 g/mol zinc 65.39 g/mol Molar Mass Examples water H2O 2(1.01) + 16.00 = 18.02 g/mol sodium chloride NaCl 22.99 + 35.45 = 58.44 g/mol Molar Mass Examples sodium bicarbonate NaHCO3 22.99 + 1.01 + 12.01 + 3(16.00) = 84.01 g/mol sucrose C12H22O11 12(12.01) + 22(1.01) + 11(16.00) = 342.34 g/mol Molar Conversions molar mass 6.02 1023 MASS NUMBER MOLES IN GRAMS OF PARTICLES (g/mol) (particles/mol) Molar Conversion Examples How many moles of carbon are in 26 g of carbon? 26 g C 1 mol C 12.01 g C = 2.2 mol C Molar Conversion Examples How many molecules are in 2.50 moles of C12H22O11? 2.50 mol 6.02 1023 molecules 1 mol = 1.51 1024 molecules C12H22O11 Molar Conversion Examples Find the mass of 2.1 1024 molecules of NaHCO3. 2.1 1024 molecules 1 mol 6.02 1023 molecules 84.01 g 1 mol = 290 g NaHCO3 Formula Calculations Percentage Composition the percentage by mass of each element in a compound mass of element % composition 100 total mass Percentage Composition Find the % composition of Cu2S. %Cu = %S = 127.10 g Cu 159.17 g Cu2S 32.07 g S 159.17 g Cu2S 100 = 79.852% Cu 100 = 20.15% S Percentage Composition Find the percentage composition of a sample that is 28 g Fe and 8.0 g O. %Fe = %O = 28 g 36 g 8.0 g 36 g 100 = 78% Fe 100 = 22% O Percentage Composition How many grams of copper are in a 38.0-gram sample of Cu2S? Cu2S is 79.852% Cu (38.0 g Cu2S)(0.79852) = 30.3 g Cu Percentage Composition Find the mass percentage of water in calcium chloride dihydrate, CaCl2•2H2O? %H2O = 36.04 g 147.02 g 100 = 24.51% H2O Empirical Formula Smallest whole number ratio of atoms in a compound C2H6 reduce subscripts CH3 Empirical Formula 1. Find mass (or %) of each element. 2. Find moles of each element. 3. Divide moles by the smallest # to find subscripts. 4. When necessary, multiply subscripts by 2, 3, or 4 to get whole #’s. Empirical Formula Find the empirical formula for a sample of 25.9% N and 74.1% O. 25.9 g 1 mol = 1.85 mol N 14.01 g 1.85 mol 74.1 g 1 mol 16.00 g = 4.63 mol O 1.85 mol =1N = 2.5 O Empirical Formula N1O2.5 Need to make the subscripts whole numbers multiply by 2 N2O5 Try Now: Empirical Formula 1. Quantitative analysis shows that a compound contains 32.38% sodium, 22.65% sulfur, and 44.99% oxygen. Find the empirical formula of this compound. Try Now: Empirical Formula 2. Analysis of a 10.150g sample of a compound known to contain only phosphorus and oxygen indicates a phosphorus content of 4.433g. What is the empirical formula of this compound? Molecular Formula “True Formula” - the actual number of atoms in a compound empirical formula CH3 ? molecular formula C2H6 Molecular Formula 1. Find the empirical formula. 2. Find the empirical formula mass. 3. Divide the molecular mass by the empirical mass. 4. Multiply each subscript by the answer from step 3. MF mass n EF mass EF n Molecular Formula The empirical formula for ethylene is CH2. Find the molecular formula if the molecular mass is 28.1 g/mol? empirical mass = 14.03 g/mol 28.1 g/mol 14.03 g/mol = 2.00 (CH2)2 C2H4 Try Now: Molecular Formula 1. In a previous example we found the empirical formula to be P2O5. Experimentation shows that the molar mass of this compound is 283.89g/mol. What is the compound’s molecular formula? Try Now: Molecular Formula 2. Determine the molecular formula of the compound with an empirical formula of CH and a formula mass of 78.110amu. Try Now: Molecular Formula 3. A sample of a compound with a formula mass of 34.00amu is found to consist of 0.44g hydrogen and 6.92g oxygen. Find its molecular formula.