* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download No Slide Title - McMaster Chemistry
Chemical equilibrium wikipedia , lookup
Asymmetric induction wikipedia , lookup
History of chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Marcus theory wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthesis wikipedia , lookup
Stoichiometry wikipedia , lookup
Organic chemistry wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Transition state theory wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Chemical reaction wikipedia , lookup
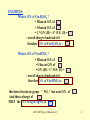
CHEMISTRY - science of properties and transformations of matter CHEMICAL REACTIONS - central to CHEMSITRY CLASSIFICATION of REACTIONS (Kotz, Ch. 4) Precipitation Oxidation-reduction Acid-base Gas forming 1A03/1E03 Types of Reactions (2) 1 OXIDATION-REDUCTION REACTIONS Cu (s) + 2 AgNO3 (aq) Cu(NO3)2 (aq) + 2 Ag(s) (see Fig. 4.17 in Kotz) Transfer of electrons: Cu0 gives 2 electrons to 2 Ag+ to form Cu2+ and 2 Ag0 species that provides electrons …….. called the REDUCING AGENT species that accepts electrons …….. called the OXIDIZING AGENT species that loses electrons is said to have been OXIDIZED species that gains electrons is said to have been REDUCED Chemists use the concept of OXIDATION NUMBER to recognize OXIDATION-REDUCTION reactions Here Cu in Cu (s) O.N. = 0 Cu in Cu(NO3)2 O.N. = +2 Ag in AgNO3 O.N. = +1 Ag in Ag(s) O.N. = 0 1A03/1E03 Types of Reactions (2) 2 RULES for OXIDATION NUMBERS O.N. (see Kotz, p. 191) • Atoms in pure ELEMENT (Cu, I2, S8) • Ions of single atom (Al#+) • Fluorine in compounds (NaF) -1 • other halogens in compounds (KCl, CsI) -1 0 charge on ion – EXCEPT when combined with F or O where O.N. = +1 • hydrogen in most compounds (HCl, HI) +1 – EXCEPT metal hydrides (CaH2) where O.N. = -1 • oxygen in most compounds (MgO, Na2O) -2 – EXCEPT in peroxides (H2O2) where O.N. = -1 • the O.N. of all other elements in a compound are determined by requiring that SUM of O.N. of all elements = CHARGE on the compound 1A03/1E03 Types of Reactions (2) 3 EXAMPLES: What is O.N. of S in H2SO4 ? • H has an O.N. of • O has an O.N. of • 2 * O.N. (H) + 4 * O.N. (O) = - overall charge of molecule is 0 therefore O.N. of S in H2SO4 is : What is O.N. of N in HNO3 ? • H has an O.N. of • O has an O.N. of • O.N. (H) + 3 * O.N. (O) = - overall charge of molecule is 0, therefore O.N. of N in HNO3 is : this shows the nitrate group: “ NO3 “ has a net O.N. of (and thus a charge) of THUS the O.N. of Ag in AgNO3 is 1A03/1E03 Types of Reactions (2) 4 Another example of an OXIDATION-REDUCTION REACTION Fe2O3 (s) + 3 CO (g) Species Fe in Fe2O3 O.N. +3 C in CO +2 2 Fe (s) + 3 CO2 (g) Fe 0 C in CO2 +4 Thus: Fe in Fe2O3 GAINS 3 electrons - Fe2O3 is the OXIDIZING AGENT C in CO LOSES 2 electrons - CO is the REDUCING AGENT The iron in Fe2O3 was REDUCED to Fe The carbon in CO was OXIDIZED to CO2 NOTES: • oxidation-reduction reactions DO NOT require ions; can involve gases • OXIDATION NUMBERS do not represent actual charge - rather they are a convenient book-keeping device which help to identify and classify reactions 1A03/1E03 Types of Reactions (2) 5 ACID-BASE REACTIONS (see alsoKotz Ch 17 - sections 1 to 5 (pp. 794-804) TYPES OF ACIDS/BASES • Arrhenius: – ACID - substance that donates H+ in water HCl H+(aq) + Cl- (aq) – BASE - substance that donates OH- in water NaOH Na+ (aq) + OH- (aq) – SALT - ionic product of an ACID - BASE reaction, composed of a +ve CATION from the base and a -ve ANION from the acid HCl (aq) + NaOH (aq) ACID + BASE NaCl (aq) + H2O SALT + 1A03/1E03 Types of Reactions (2) water 6 Brnsted - Lowry: ( sect 17.3, p. 707) ACID - substance that donates H+ to another species HCO3- (aq) H+(aq) + CO32- (aq) BASE - substance that accepts H+ from another species NH3 + H2O NH4+ (aq) + OH- (aq) TYPICAL PROPERTIES of ACIDS and BASES ACIDS • sour tase •corrosive •reacts with bases •turns natural dyes red •generates CO2 from limestone •generates H2 with metals BASES •soapy feel •restores natural dyes to blue •reacts with acids 1A03/1E03 Types of Reactions (2) 7 CONJUGATE ACID-BASE PAIRS A pair of compounds that differ in composition by one H+ - H+ + H+ HBr (aq) + NH3 (aq) NH4+ (aq) + ACID BASE conjugate ACID of NH3 Br- (aq) conjugate BASE of HBr ALL ACID-BASE REACTIONS involve TWO CONJUGATE ACID-BASE PAIRS Acid(1) + HCl + H2 O + CH3CO2H + Base(2) NH3 NH3 H2 O Conj-Acid(2) + NH4+ NH4+ H3 O+ 1A03/1E03 Types of Reactions (2) + + + Conj-Base(1) ClOHCH3CO28 RELATIVE STRENGTHS of ACIDS and BASES STRONG ACIDS - react completely with water to form H3O+ (aq) HCl (aq) + H2O H3O+ (aq) + Cl- (aq) STRONG BASES - react completely with water to form OH- (aq) Li2O + H2O 2 Li+ (aq) + OH- (aq) Weak ACIDS/ weak BASES only react partially with water - an EQUILIBRIUM is formed : the conjugate ACID and the conjugate BASE are both present at the same time WEAK ACID: (acetic acid a.k.a. vinegar) CH3CO2H + H2O CH3CO2- (aq) + H3O+ (aq) WEAK BASE: NH3 (g) + H2O NH4+ (aq) + OH- (aq) 1A03/1E03 Types of Reactions (2) 9 Relative Strengths of acids/bases depend on the solvent : H3O+ is the STRONGEST ACID OH- is the STRONGEST BASE Once an acid or base is fully reacted (dissociated) to form these species, one can no longer distinguish relative strengths. This is called the LEVELLING ACTION of water (The relative strengths of two strong acids can only be determined in non-aqueous solvents) In water: STRONG ACIDS: WEAK ACIDS: HCl, H2SO4, H3PO4, HNO3, HClO4 CH3CO2H, “H2CO3” (= CO2), HS, HCN, HF WEAK BASES: NH3, Na2CO3 STRONG BASES: NaOH, Li2O, NaH, Ca(OH)2 Kotz, Table 17.3 In Br nsted acid-base reactions - H+ transfer occurs from STRONGER to WEAKER congugate acid-base pair 1A03/1E03 Types of Reactions (2) 10 GAS FORMING REACTIONS - some reactions generate gases. When carried out in an OPEN system, the escape of the gas acts as a DRIVING FORCE for the chemical reaction - loss of gas product pulls reaction to completion just as formation of a precipitate e.g. CaCO3 (s) + 3 HCl (aq) Zn (s) + 2 HCl (aq) CaCl2 (aq) + H2O (l) + CO2 (g) ZnCl2 (aq) + H2 (g) NB. Each of these reactions can also be classified as one of the other 3 types of reactions - WHICH ONES ? 1A03/1E03 Types of Reactions (2) 11 GAS FORMING REACTIONS - alternative classification A. CaCO3 (s) + 3 HCl (aq) CaCl2 (aq) + H2O (l) + CO2 (g) There is no precipitate and no change in O.N. (Ca stays as +2, Cl as -1) It is an ACID-BASE REACTION involving an intermediate H2CO3 called CARBONIC ACID, which immediately dissociates to H2O and CO2 2 H+ + CO32- “H2CO3 “ H2O + CO2 B. Zn (s) + 2 HCl (aq) ZnCl2 (aq) + H2 (g) There is no precipitate. However there is an obvious change in O.N. It is also an example of an OXIDATION-REDUCTION REACTION Zn goes from O.N. = 0 in the metal to O.N. = +2 in the chloride salt H goes from O.N. = +1 in HCl (aq) to O.N. = 0 in the elemental gas 1A03/1E03 Types of Reactions (2) 12 CHEMISTRY of HALOGENS The Elements • Group 7A - all non-metals • diatomc molecules: F2 (gas), Cl2 (gas), Br2 (liquid), I2 (solid) • most stable oxidation state: -1 • Undergo OXIDATION-REDUCTION reactions readily with metals (all elements on right and middle of P.T.) Trends: SIZE: Electron affinity: Oxidizing strength I > Br > Cl > F F > Cl > Br > I F > Cl > Br > I Compounds most stable form: HALIDES (A+X-) hydrogen halides: ACIDS: HCl (strong), others (weak) 1A03/1E03 Types of Reactions (2) 13 CHLORINE prepared by electrochemical reaction (chlor-alkali process) ELECTROLYSIS 2 NaCl + H2O -------> Cl2 + 2 NaOH • 8th most important industrial chemical by mass (1010 kg/yr) IMPORTANT USES • disinfectant of water (as hpyochlorous acid (HOCl(aq)) Cl2 + 2 H2O H3O+ + Cl- + HOCl (aq) • bleach for textiles, paper, etc (as hypochlorite (OCl-) Cl2 + 2 OH- H2O + Cl- + OCl- (aq) • organochlorine compounds: plastics, drugs 1A03/1E03 Types of Reactions (2) 14 CHLORINE: BANE or BOON ??? Kotz, pp 88,89 Consumer friend . . . . . . . or environmental foe ?? 1A03/1E03 Types of Reactions (2) 15 SUMMARY: Key Concepts from “Types of Reactions” (Kotz Ch. 4) •Classes of reactions: precipitation oxidation-reduction acid-base gas-forming •Ionic versus non-ionic compounds •Solubility guidelines: Kotz, p. 168 •Oxidation Number rules: Kotz, p. 191 •Acid/base types: Arrhenius, Br nsted- Lowry • Typical chemistry of halogens •Some reactions PROCEED TO COMPLETION e.g. precipitation, gas forming, strong acid/strong base •Some reactions establish an EQUILIBRIUM of reactants and products e.g. oxidation-reduction, weak acids, weak bases LEARN TO BALANCE CHEMICAL EQUATIONS - both MASS and CHARGE balance are important 1A03/1E03 Types of Reactions (2) 16 CHEMISTRY of DEMONSTRATIONS Oxidation-Reduction iron and chlorine: 2 Fe (s) + 3 Cl2 (g) 2 FeCl3 (s) (black) sugar and sulfuric acid: 2C6H12O6 (s) + 12 H2SO4 (aq) 6C (s) + 6 CO2 (g) + 24 H2O (l) + 12 SO2 (g) Acid-Base acid and limestone: 2 H+ (aq) + CaCO3 (s) Ca2+ (aq) + CO2 (g) + H2O(l) acid and metal: 2 H+ (aq) + Zn (s) Zn2+ (aq) + H2 (g) 1A03/1E03 Types of Reactions (2) 17