* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Physical Properties
Survey
Document related concepts
Physical organic chemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Water testing wikipedia , lookup
Properties of water wikipedia , lookup
Atomic theory wikipedia , lookup
Water splitting wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Implicit solvation wikipedia , lookup
Water pollution wikipedia , lookup
Solvent models wikipedia , lookup
Crystallization wikipedia , lookup
Electrolysis of water wikipedia , lookup
Transcript
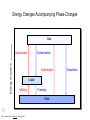
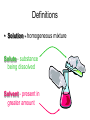
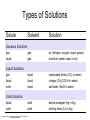
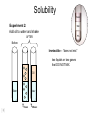
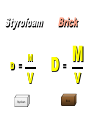
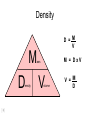
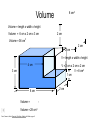
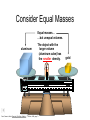
Basic Concepts in Chemistry Physical Properties Physical Properties A characteristic of a substance that you can observe and measure without changing the identity of the substance. Number vs. Quantity • Quantity - number + unit UNITS MATTER!! Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Types of measurement Quantitative- can be measured and assigned a particular value. use numbers to describe - melting point - viscosity is a measure of how easily a fluid flows. - 4 meters, - 100 0C Qualitative- can be observed and described without detailed - measurements. the colour of aluminum is grey. Hydrogen sulfide has a characteristic odour of rotten eggs. Greenish-yellow gas could be a warning that chlorine gas is present. extra large hot Scientists prefer Quantitative - easy to check • Easy to agree upon, no personal bias • The measuring instrument limits how good the measurement is. Examples of Qualitative Physical Properties Property Examples Colour Colourless, red, black Odour Sweet, pungent, mouldy State Solid, liquid, or gas Texture Rough, smooth, bumpy Lustre Shiny, dull malleability Soft, pliable, hard Quantitative Physical Properties Property Description Viscosity Resistance to flow Melting point Temperature of melting Boiling point Temperature of boiling Solubility Ability to dissolve in another substance Hardness Ability to scratch another material Conductivity Ability to conduct electricity or heat Density Ratio of mass to volume States of Matter Gas, Liquid, and Solid Gas Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 441 Liquid Solid Some Properties of Solids, Liquids, and Gases Property Solid Liquid Gas Shape Has definite shape Takes the shape of the container Takes the shape of its container Volume Has a definite volume Has a definite volume Fills the volume of the container Arrangement of Particles Fixed, very close Random, close Random, far apart Interactions between particles Very strong Strong Essentially none Evaporation • To evaporate, molecules must have sufficient energy to break Intermolecular (IM) forces. Change of state from a liquid to a gas. • Molecules at the surface break away and become gas. • Only those with enough Kinetic energy (KE) escape. • Breaking IM forces requires energy. The process of evaporation is endothermic. • Evaporation is a cooling process. • It requires heat. Condensation Change from gas to liquid Achieves a dynamic equilibrium with vaporization in a closed system. What is a closed system? A closed system means matter can’t go in or out. (put a cork in it) Energy Changes Accompanying Phase Changes Gas Energy of system Vaporization Condensation Sublimation Liquid Melting Freezing Solid Brown, LeMay, Bursten, Chemistry 2000, page 405 Deposition Melting / Boiling Point Celsius Boiling point of water Freezing point of water Absolute zero 100oC 100 Celsius degrees 0oC -273oC Melting Point change of state from solid to liquid Melting point: the temperature at which a solid turns into a liquid. Boiling point: the temperature at which a liquid turns into a gas. Boiling point of water is 100 0C. Definitions • Solution - homogeneous mixture Solute - substance being dissolved Solvent - present in greater amount Solutions • What the solute and the solvent are determines –whether a substance will dissolve. –how much will dissolve. • A substance dissolves faster if it is stirred or –The particles are made smaller. shaken. –The temperature is increased. Why? Solution = Solute + Solvent • Solute - gets dissolved • Solvent - does the dissolving – Aqueous – Tincture – Amalgam – Organic • Polar • Non-polar (water) (alcohol) (mercury) Dental filling Nightmare on White Street Chem Matters, December 1996 Solution Definitions solution: a homogeneous mixture -- evenly mixed at the particle level -- e.g., salt water alloy: a solid solution of metals -- e.g., bronze = Cu + Sn; brass = Cu + Zn solvent: the substance that dissolves the solute water salt soluble: “will dissolve in” miscible: refers to two gases or two liquids that form a solution; more specific than “soluble” -- e.g., food coloring and water Types of Solutions Solute Solvent Solution gas gas air (nitrogen, oxygen, argon gases) humid air (water vapor in air) liquid liquid liquid carbonated drinks (CO2 in water) vinegar (CH3COOH in water) salt water (NaCl in water) solid solid dental amalgam (Hg in Ag) sterling silver (Cu in Ag) Gaseous Solutions gas liquid Liquid Solutions gas liquid solid Solid Solutions liquid solid Charles H.Corwin, Introductory Chemistry 2005, page 369 Factors Affecting the Rate of Dissolution 1. temperature 2. particle size 3. mixing 4. nature of solvent or solute As To , rate As size , rate More mixing, rate Classes of Solutions aqueous solution: solvent = water water = “the universal solvent” amalgam: solvent = Hg e.g., dental amalgam tincture: solvent = alcohol e.g., tincture of iodine (for cuts) organic solution: solvent contains carbon e.g., gasoline, benzene, toluene, hexane Non-Solution Definitions insoluble: “will NOT dissolve in” e.g., sand and water immiscible: refers to two gases or two liquids that will NOT form a solution e.g., water and oil suspension: appears uniform while being stirred, but settles over time Solubility Experiment 1: Add 1 drop of red food coloring Before AFTER Miscible – “mixable” two gases or two liquids that mix evenly Water Water Water Water COLD HOT COLD HOT B A B A Solubility Experiment 2: Add oil to water and shake AFTER Before Immiscible – “does not mix” two liquids or two gases that DO NOT MIX Oil Water Water T0 sec T30 sec Solubility vs. Temperature Solubility (g solute / 100 g H2O) 200 180 160 140 120 100 80 60 NaCl 40 20 0 20 40 60 Temperature (oC) Timberlake, Chemistry 7th Edition, page 297 80 100 Pure water does not conduct an electric current Source of electric power Pure water Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 215 Ionic Solutions conduct a Current Source of electric power Free ions present in water Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 215 Electrolytes (a) Nonelectrolyte Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. (b) Weak electrolyte (c) Strong electrolyte Electrolytes Electrolytes - solutions that carry an electric current strong electrolyte NaCl(aq) Na+ + Cl- Timberlake, Chemistry 7th Edition, page 290 weak electrolyte HF(aq) H+ + F- nonelectrolyte Solubility and the Environment Chemical Stewardship • Be responsible in how you dispose of and use chemicals. • Chemical pollution can travel far – and harm organisms. Frog with three legs – it has mutated from chemical exposure. Early Warning Signs… = Contamination of water where frog lives causes mutations. Canary dies in the mine from bad air (cyanide). This is clue for miners to leave the mine…alive. Pfiesteria Pfiesteria organism Fish sores from infection by pfiesteria. Solvents Solvents at the hardware store Density • Density is an INTENSIVE property of matter. - does NOT depend Brick Styrofoam on quantity of matter. - color, melting point, boiling point, odor, density • Contrast with EXTENSIVE - depends on quantity of matter. - mass, volume, heat content (calories) It appears that the brick is ~40x more dense than the Styrofoam. Styrofoam ? Brick Styrofoam D = Styrofoam M V Brick D = Brick M V Which liquid has the highest density? least dense 1 < 3 < 5 < 2 1 3 2 Coussement, DeSchepper, et al. , Brain Strains Power Puzzles 2002, page 16 5 4 < 4 most dense Cube Representations 1 m3 = 1 000 000 cm3 Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 119 Volume and Density Relationship Between Volume and Density for Identical Masses of Common Substances Substance Cube of substance (face shown actual size) Mass (g) Volume (cm3) 19 Density (g/cm3) Lithium 10 0.53 Water 10 10 1.0 Aluminum 10 3.7 2.7 Lead 10 0.58 11.4 Density D = M V M M = DxV ass D ensity V olume V = M D Volume 4 cm 3 cm 1 cm 2 cm 6 cm Dorin, Demmin, Gabel, Chemistry The Study of Matter 3rd Edition, page 41 8 cm3 Volume Volume = length x width x height Volume = 6 cm x 2 cm x 3 cm 2 cm Volume = 36 cm3 2 cm 2 cm V = length x width x height 4 cm V = 2 cm x 2 cm x 2 cm V = 8 cm3 1 cm 3 cm 2 cm 6 cm Volume = Volume = 28 cm3 Dorin, Demmin, Gabel, Chemistry The Study of Matter 3rd Edition, page 41 - Density of Some Common Substance Density of Some Common Substances Substance Density (g / cm3) Air Lithium Ice Water Aluminum Iron Lead Gold *at 0oC and 1 atm pressure 0.0013* 0.53 0.917 1.00 2.70 7.86 11.4 19.3 Consider Equal Volumes Mass Density = Volume Equal volumes… …but unequal masses The more massive object (the gold cube) has the GREATER _________ density. aluminum Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 71 gold Consider Equal Masses Equal masses… …but unequal volumes. aluminum The object with the larger volume (aluminum cube) has the smaller density. gold Christopherson Scales Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 71 (A) Equal volumes… …but unequal masses Two ways of viewing density The more massive object (the gold cube) has the greater density. (B) Equal masses… …but unequal volumes. aluminum Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 71 gold aluminum The object with the larger volume (aluminum cube) has the smaller density. gold Water Molecule Water is a POLAR molecule d+ H2O d- d+ H+ H+ O2- d- Water molecules “stick” together to create surface tension to support light weight objects. Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. Water Molecule • What is a polar molecule? dHydrogen bond d+ H • How does the polarity of water effect this molecule? O H • Hydrogen bonds occur between two polar molecules, or between different polar regions of one large macromolecule. • One “relatively” negative region is attracted to a second “relatively” positive region. H O Electronegative atoms H Hydrogen bond N H H H Reviewing Concepts Physical Properties • List seven examples of physical properties. • Describe three uses of physical properties. • Name two processes that are used to separate mixtures. • When you describe a liquid as thick, are you saying that it has a high or low viscosity? Reviewing Concepts Physical Properties • Explain why sharpening a pencil is an example of a physical change. • What allows a mixture to be separated by distillation?