* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Student notes in ppt
Survey
Document related concepts
Basal metabolic rate wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Lipid signaling wikipedia , lookup
Citric acid cycle wikipedia , lookup
Signal transduction wikipedia , lookup
Paracrine signalling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Human digestive system wikipedia , lookup
Biochemistry wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Transcript
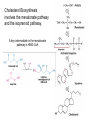
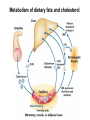
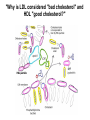
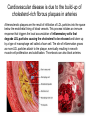
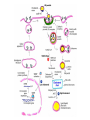
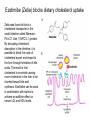
Lipid Metabolism 3: Cholesterol biosynthesis, lipoprotein metabolism, steroid and eicosanoid synthesis Bioc 460 Spring 2008 - Lecture 37 (Miesfeld) Mike Brown and Joe Goldstein shared the 1985 Nobel Prize for their work on cholesterol metabolism Cardiovascular disease is caused by fatty acid deposits that sequester cholesterol in plaques that can break off Steroids are cholesterol derivatives that have been used as anabolic enhancers Key Concepts in Lipid Metabolism • Cholesterol is major component of membranes that is synthesized in liver cells from acetyl-CoA through a complex reaction pathway involving six C5 intermediate isopentenyl pyrophosphate that form a C30 squalene product. • Cholesterol metabolism is an important component of cardiovascular disease because of its association with the formation of atherosclerotic plaques. Over 70% of serum cholesterol is synthesized in the liver which is why statin drugs target the cholesterol biosynthetic pathway. • Steroid hormones are cholesterol derivatives that bind to nuclear receptor proteins in cells that function as gene-specific transcription factors. Steroid hormones are important physiological ligands and pharmaceutical drugs. • Eicosanoids are fatty acid derivatives that are precursors to paracrine signaling molecules such as prostaglandins, thromboxanes, and leukotrienes, all of which are drug targets for various inflammatory diseases. Cholesterol Biosynthesis Cholesterol is a large hydrophobic molecule that controls membrane fluidity in eukaryotes and is a metabolic precursor to steroid hormones. The majority of serum-derived cholesterol comes from de novo biosynthesis in the liver, not from dietary cholesterol. Therefore, understanding cholesterol biosynthesis in relation to cardiovascular disease has been a very active research area. Radioisotope labeling experiments have shown that all 27 carbon atoms of cholesterol are derived from acetyl-CoA. Cholesterol Biosynthesis involves the mevalonate pathway and the isoprenoid pathway. A key intermediate in the mevalonate pathway is HMG-CoA Cholesterol biosynthesis takes place in the cytosol and consists of four distinct stages In the first stage, three molecules of acetyl-CoA are used to make mevalonate, a C6 compound that is the product of a reaction catalyzed by the highly-regulated enzyme HMG CoA reductase. Mevalonate is then phosphorylated and decarboxylated in stage 2 to form the activated C5 isoprenoid intermediate, isopentenyl pyrophosphate. This important metabolite is an intermediate in a number of other biosynthetic reactions including those of plant chlorophylls and plant hormones. In stage 3, three molecules of isopentenyl pyrophosphate are combined to form farnesyl pyrophosphate (C15) which is then used to generate squalene, a C30 cholesterol precursor. In the fourth and final stage, squalene cyclicizes to form a four-ringed molecule which is then modified by a number of reactions, ultimately resulting in the loss of three methyl groups to generate cholesterol (C27), a four-ringed sterol molecule. Stage 1: Generation of mevalonate from acetyl-CoA In the first reaction of this cytosolic pathway, two molecules of acetyl-CoA are condensed to form acetoacetyl-CoA through a reaction catalyzed by the enzyme thiolase. A third acetyl-CoA is used to generate the C6 compound Hydroxy--methylglutaryl-CoA (HMGCoA) by the action of HMG-CoA synthase. In what turns out to be the rate-limiting step in the cholesterol biosynthetic pathway, the enzyme HMGCoA reductase converts HMG-CoA to mevalonate in a reduction reaction that uses two molecules of NADPH and releases coenzyme A. Stage 2: Conversion of mevalonate to isopentenyl pyrophosphate and dimethylallyl pyrophosphate Mevalonate is activated by the addition of two phosphates groups donated from ATP to generate 5-pyrophosphomevalonate. The enzyme pyrophosphomevalonate decarboxylase then catalyzes an ATP-dependent reaction that removes the terminal carboxyl group to generate the C5 isoprenoid compound isopentenyl pyrophosphate which is readily isomerized to form dimethylallyl pyrophosphate. isopentenyl pyrophosphate dimethylallyl pyrophosphate Stage 2: Conversion of mevalonate to isopentenyl pyrophosphate and dimethylallyl pyrophosphate 1 C6 1 C5 isomerize or 1 C5 Stages 3 and 4: formation of cholesterol (C27) from six C5 isoprenoids Prenyl transferase catalyzes a condensation reaction in which isopentenyl pyrophosphate and dimethylallyl pyrophosphate condense in a head to tail fashion to form the C10 compound geranyl pyrophosphate. The same enzyme adds a second isopentenyl pyrophosphate to generate farnesyl pyrophosphate. Two molecules of farnesyl pyrophosphate are then linked in a head to head arrangement by the enzyme squalene synthase to form squalene (C30) in a reduction reaction using NADPH. The conversion of a C30 hydrocarbon chain into a cyclic cholesterol molecule involves a complex set of reactions that are catalyzed by enzymes called cyclases that promote the formation of the four-ringed cholesterol precursor lanosterol. In the final steps of the pathway, lanosterol is converted to cholesterol by a series of 19 reactions that involve additional bond rearrangements, and ultimately, the removal of three carbons to generate the C27 cholesterol product. 1 C5 Stages 3 and 4: formation of cholesterol (C27) from six C5 isoprenoids + 1 C5 1 C30 1 C10 +1 C5 1 C15 +1 C15 1 C27 1 C30 Cholesterol synthesized in the liver has three metabolic fates; stored, exported, or converted to bile acid Cholesterol can be esterified with a fatty acid by the enzyme acyl-CoAcholesterol acyl transferase (ACAT) to make cholesterol esters that are stored in lipid droplets, or packaged into lipoprotein particles and exported to the peripheral tissues. As much as 50% of the cholesterol synthesized in liver cells on a daily basis is converted to bile acids which are transported to the bile duct and secreted into the small intestine to aid in fat absorption. Cholesterol synthesized in the liver has three metabolic fates; stored, exported, or converted to bile acid What would happen to cholesterol levels in the liver if bile acids were excreted rather than recycled back to the liver? Bile acid resin Metabolism of dietary fats and cholesterol Triacylglycerols and cholesterol are transported through the circulatory system as components of plasma lipoprotein particles that consist of membrane-bound vesicles containing a hydrophobic core and one or more proteins on the surface called apolipoproteins. Metabolism of dietary fats and cholesterol Chylomicrons contain mostly triacylglycerols in their hydrophobic core and a low amount of protein relative to lipid, thereby giving them the lowest density. In contrast, high density lipoproteins (HDL), have a high percentage of protein compared to lipid and are very small resulting in high density. The three other lipoprotein classes, very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL), also called VLDL remnants, and low density lipoproteins (LDL), are progressively smaller and contain higher ratios of protein to lipid. Metabolism of dietary fats and cholesterol Lipoproteins function in the body to transport lipids (triacylglycerols and cholesterol) from the small intestine or the liver out to peripheral tissues and then back again to the liver. The level of lipoproteins in blood plasma is significant after a lipid-rich meal as evidenced by the milky appearance of blood due to the high numbers of chylomicrons. Cholesterol deposits in blood vessels are considered one of the major culprits in cardiovascular disease, and therefore, control of cholesterol homeostasis is an important regulatory process. Metabolism of dietary fats and cholesterol Steady state levels of serum cholesterol are determined by cholesterol recycling via lipoprotein particles, and by cholesterol excretion. Lipoproteins, primarily VLDL particles, transport the triacylglycerol and cholesterol synthesized in liver cells (or deposited there by the chylomicron remnants) out to the peripheral tissues. Like chylomicrons, VLDL particles contain apoC-II and ApoE on the surface to facilitate triacylglycerol delivery to the tissues, but they also contain apolipoprotein B-100, an apolipoprotein that is recognized by another cell surface receptor called the LDL receptor. About half of the VLDL particles are converted to IDL particles (VLDL remnants) that are returned to the liver, and the rest continue to circulate until nearly all of the triacylglycerol is removed and they are converted to LDL particles. These cholesterol-rich lipoproteins are called "bad cholesterol" and are Metabolism of dietary fats and cholesterol "Why is LDL considered "bad cholesterol" and HDL "good cholesterol?" The answer is that high LDL levels in the serum are clinically associated with an increased risk of atherosclerosis, whereas, high HDL levels in the serum are associated with a significantly decreased risk of atherosclerosis. Therefore it is "bad" to have too much LDL in the serum and "good" to have elevated levels of HDL. "Why is LDL considered "bad cholesterol" and HDL "good cholesterol?" Cardiovascular disease is due to the build-up of cholesterol-rich fibrous plaques in arteries Atherosclerotic plaques are the result of infiltration of LDL particles into the space below the endothelial lining of blood vessels. This process initiates an immune response that triggers the local accumulation of inflammatory cells that degrade LDL particles causing the cholesterol to be released and taken up by a type of macrophage cell called a foam cell. The site of inflammation grows as more LDL particles attach to the plaque, eventually resulting in smooth muscle cell proliferation and calcification. Thrombosis can also block arteries. LDL receptor cycling and cholesterol homeostasis The connection between high serum LDL levels and atherosclerosis was made by Brown and Goldstein when they discovered that a genetic disorder called familial hypercholesterolemia (FH) was due to mutations in the LDL receptor gene. The cholesterol esters are released from lysosomes and stored in cholesterol droplets or used to synthesize bile acids or steroids. The intracellular pool of cholesterol is determined by both cholesterol ester uptake from LDL particles and de novo cholesterol biosynthesis. Low cholesterol levels stimulate HMG-CoA reductase activity and induce expression of the LDL receptor gene through protealytic activation of sterol regulatory element binding proteins (SREBPs) that are transcription factors that regulate the expression of numerous lipid metabolizing genes. Statin drugs inhibit cholesterol biosynthesis by blocking the activity of HMG-CoA reductase Since de novo synthesis of liver cholesterol accounts for the majority of cholesterol in our bodies (~70% for most individuals), pharmaceutical companies first developed drugs that could be used to inhibit cholesterol synthesis and thereby activate LDL receptor expression. The result of this treatment strategy is decreased serum LDL levels due to uptake by liver cells; decreased serum LDL levels is associated with reduced risk of cardiovascular disease. 4 How do statin drugs lower serum LDL levels? Why would this decrease the risk of cardiovascular disease? 1 3 2 Ezetimibe (Zetia) blocks dietary cholesterol uptake Zetia was found to block a cholesterol transporter in the small intestine called NiemannPick C1 Like 1 (NPC1L1) protein. By disrupting cholesterol absorption in the intestine, it is possible to block the cycle of cholesterol export and import to the liver through formation of bile acids. The result is that cholesterol is excreted causing more cholesterol in the liver to be shunted toward bile acid synthesis. Ezetimibe can be used in combination with statins to achieve an additive effect on serum LDL and HDL levels. Ezetimibe (Zetia) blocks dietary cholesterol uptake A cholesterol-lowering drug of this type is Vytorin which contains both ezetimibe and simvastatin and is capable of lowering serum LDL levels by 50% and increasing HDL levels by as much as 10%. Vytorin contains ezetimibe and simvastatin. Surprisingly, recent clinical studies have shown that Vytorin, a Merck collaborative drug, is no more effective in reducing cardiovascular disease than statin drugs alone, eventhough Vytorin lowers serum cholesterol levels much more than either ezetimibe or simvastatin. It is not clear what these new results mean except that serum cholesterol levels alone may not be the best predictor of cardiovascular disease. Steroid hormones are synthesized from cholesterol The five major hormones in mammals are all derived from modifications of cholesterol following removal of the side chain at the C-17 carbon of the D ring to produce pregnenolone. This C21 sterol gives rise to progesterone which can be converted to cortisol, androstenedione and corticosterone. Cortisol is a glucocorticoid hormone that regulates liver metabolism. Androstenedione is an androgenic hormone that gives rise to testosterone which is then converted to either estradiol or dihydrotesterone. Aldosterone, a mineralocorticoid that regulates ion transport in the kidney, is derived from corticosterone. Steroid hormones are synthesized from cholesterol The three other major sites of steroid synthesis are the ovaries in females (estrogen), testes in males (testosterone), and the corpus luteum in pregnant females (progesterone). Importantly, the adrenal glands also synthesizes androgens, which is how females acquire testosterone for estradiol production. The chemical and molecular structure of steroid hormones, as well as, the structure of the steroid hormone binding domain of a steroid receptor protein, are shown below. Note the similar structure to cholesterol and the hydrophobic nature of these important signaling molecules. Steroid hormones are synthesized from cholesterol Subtle changes in the side groups of steroid hormones account for differences in their agonist (activating) or antagonist (inhibiting) functions as pharmacological agents. Dihydrotestosterone is a naturally occurring androgen, whereas, nandrolone is a potent synthetic agonist that has been abused by bodybuilders to gain muscle mass. Finasteride, a testosterone antagonist used to treat male pattern baldness, a condition caused by high levels of testosterone in the hair follicle cells. Eicosanoids Biosynthesis Eicosanoids are a group of signaling molecules derived from C20 polyunsaturated fatty acids such as arachidonate that are released from the membrane by phospholipases. The three major classes of arachidonate-derived eicosanoids are prostaglandins, thromboxanes and leukotrienes. COX-2 inhibitors COX-1 is involved in producing prostaglandins that stimulate mucin secretion and protect the lining of the stomach from high pH, COX-2 expression is specifically induced by inflammatory signals. Aspirin, ibuprofen, and naproxen, are all non-selective NSAIDs that inhibit both COX-1 and COX-2. Vioxx and Celebrex selectively inhibit COX-2, but they also partially inhibit prostacyclin synthesis which can lead to clot formation. Vioxx is no longer available because of an increased risk of heart attacks.