* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Psoriasis - New England Journal of Medicine
Survey
Document related concepts
Monoclonal antibody wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adaptive immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Innate immune system wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Transcript
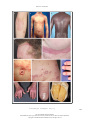
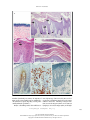
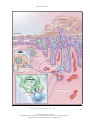
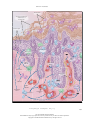
The new england journal of medicine review article medical progress Psoriasis Michael P. Schön, M.D., and W.-Henning Boehncke, M.D. p soriasis, a common inflammatory skin disorder, has received attention as a target for new pathogenesis-oriented biologic therapies. In this article, we review the genetic, clinical, and pathogenic aspects of psoriasis and discuss their implications for new therapies. epidemiologic and genetic features Though psoriasis is a common skin disease, its definition by Ferdinand von Hebra as a distinct entity dates back only to the year 1841, and estimates of its prevalence — around 2 percent, according to standard textbooks — stem from only a few population-based studies. Perhaps the most comprehensive field study was performed in the Faroe Islands, where 2.8 percent of the inhabitants were reported to be affected.1 This prevalence rate is higher than that in central Europe, where prevalence is approximately 1.5 percent, according to a more recent analysis.2 Ethnic factors also appear to influence the prevalence of psoriasis, which ranges from no cases in the Samoan population to 12 percent in Arctic Kasach’ye.2 The influence of ethnic factors is particularly evident when one compares prevalence rates within the United States. The prevalence among blacks (0.45 to 0.7 percent)3 is far lower than that in the remainder of the U.S. population (1.4 to 4.6 percent).4 Numerous family studies have provided compelling evidence of a genetic predisposition to psoriasis, although the inheritance pattern is still unclear.5 The illness develops in as many as half of the siblings of persons with psoriasis when both parents are affected, but prevalence falls to 16 percent when only one parent has psoriasis and to 8 percent when neither parent is affected.6 The concordance rate for monozygotic twins is around 70 percent, as compared with some 20 percent for dizygotic twins, a finding that further supports the concept of genetic predisposition.7,8 As many as 71 percent of patients with childhood psoriasis have a positive family history.9 Within the past decade, several putative loci for genetic susceptibility to the disease have been reported on the basis of genome-wide linkage studies, but there has not been widespread replication of the results — a problem that has also been encountered in the investigation of other complex diseases.10,11 However, one locus in the majorhistocompatibility-complex (MHC) region on chromosome 6 has been replicated in several populations12,13 (see Glossary for definitions of terms). This locus, termed psoriasis susceptibility 1 (PSORS1), is considered the most important susceptibility locus. On the basis of association studies of three tightly linked susceptibility alleles (HLA-Cw6,14 HCR*WWCC,15 and the HLA-associated S gene16), PSORS1 appears to be associated with up to 50 percent of cases of psoriasis. Other susceptibility loci are located on chromosomes 17q25 (PSORS2),17 4q34 (PSORS3),18 1q (PSORS4),19 3q21 (PSORS5),20 19p13 (PSORS6),21 and 1p (PSORS7).22 Most recently, an additional gene locus for psoriasis susceptibility has been discovered on chromosome 17q25.23 This locus, a runt-related transcription factor 1 (RUNX1) binding-site variant, encodes for a n engl j med 352;18 www.nejm.org From the Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, and the Department of Dermatology, University of Würzburg, Würzburg (M.P.S.); and the Department of Dermatology, University of Frankfurt, Frankfurt (W.-H.B.) — both in Germany. Address reprint requests to Dr. Schön at the Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine and Department of Dermatology, Julius Maximilians University, Versbacher Str. 9, 97078 Würzburg, Germany, or at michael.schoen@virchow. uni-wuerzburg.de. N Engl J Med 2005;352:1899-912. Copyright © 2005 Massachusetts Medical Society. may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1899 The new england journal Glossary CC chemokines: A subfamily of small chemoattractant peptides that have two N-terminal cysteine residues adjacent to each other. Cutaneous lymphocyte-associated antigen (CLA): A selectin ligand bearing the sialyl-Lewis X carbohydrate moiety; expressed preferentially by skin-homing lymphocytes. CXC chemokines: A subgroup of leukocyte-attracting chemokines with a different amino acid between the two N-terminal cysteine residues. Intercellular adhesion molecule 1 (ICAM-1): Also known as CD54, an adhesion molecule of the immunoglobulin superfamily functioning as a counterreceptor for adhesion molecules of the integrin family; expressed on endothelial cells and some activated epithelial cells. Interferon-g: A cytokine produced primarily by T lymphocytes, characteristic for immune responses dominated by type 1 helper T (Th1) cells, including psoriasis. Leukocyte-function–associated antigen (LFA): CD11a–CD18, a heterodimeric adhesion molecule of the integrin family expressed by lymphocytes; binds to ICAM-1, ICAM-2, and ICAM-3. Major-histocompatibility-complex (MHC) molecules: Surface molecules important for antigen presentation to T lymphocytes. PSORS: Psoriasis-susceptibility locus, one of several genomic sequences associated with psoriasis. SCID mice: Mice with severe combined immunodeficiency due to a spontaneous mutation and lacking functional B and T lymphocytes; used for transplantation and immunologic transfer studies. Transforming growth factor a (TGF-a): A cytokine involved in some tissue alterations in psoriatic skin. Vascular cell adhesion molecule 1: Also known as CD106; a vascular adhesion molecule of the immunoglobulin superfamily, a counterreceptor for the a4b1 integrin. Vascular endothelial growth factor (VEGF): A key regulator of angiogenesis, overexpressed in psoriatic skin. gene involved in the development of blood cells, including those of the immune system. clinical and histopathological features People with psoriasis typically have sharply demarcated chronic erythematous plaques covered by silvery white scales, which most commonly appear on the elbows, knees, scalp, umbilicus, and lumbar area (Fig. 1A to 1D). An inverse type of psoriasis spares these sites and instead appears in intertriginous areas, where scaling is minimal (Fig. 1E). Eruptive guttate psoriasis, which may be the initial manifestation of the disease and is often preceded by streptococcal infection by two to three weeks,24 exhibits small, disseminated erythematosquamous papules and plaques (Fig. 1B). Psoriatic diaper rash appears to be the most common type of psoriasis in children under the age of two years.9 In addition to small pustules that may occur in 1900 n engl j med 352;18 of medicine Figure 1 (facing page). Clinical Features of Psoriasis. The typical psoriatic lesion is a sharply demarcated erythematous plaque covered by silvery white scales, often appearing on the elbow (Panel A). Initial eruptions of psoriasis may exhibit a guttate distribution pattern and are often triggered by streptococcal infections (Panel B). In a dark-skinned patient, erythrodermic psoriasis (Panel C), a clinical subtype of the disease, affects the entire body surface. Psoriatic erythroderma may arise from any type of psoriasis. Scalp involvement is seen in approximately 50 percent of patients with psoriasis, and the lesions typically extend a short distance beyond the region covered by terminal hair (Panel D). Inverse psoriasis (Panel E) is located at intertriginous areas and usually shows only scant scaling. In patients with psoriasis vulgaris, small sterile pustules may develop (Panel F, which is a magnification of the periumbilical region from Panel B). Generalized pustular psoriasis may start from coalescing disseminated pustules on deeply erythematous skin (Panel G). Localized forms of psoriasis include acrodermatitis continua suppurativa, or Hallopeau’s disease (Panel H, which shows the fingers of a patient with severe onychodystrophy). Approximately 5 to 20 percent of patients with psoriasis have psoriatic arthritis (Panel I). Nail involvement is frequent in patients with psoriasis, and mild cases are characterized by small pits and yellowish discoloration of the nail plate (Panel J). Psoriatic nail pits were recreated in a wax-model moulage manufactured approximately 100 years ago (Panel K, which is item 1766 from the collection of the Johann Wolfgang Goethe University, Frankfurt, Germany). lesions of psoriasis vulgaris, various forms of pustular psoriasis have been described (Fig. 1F). Generalized pustular psoriasis (Fig. 1G) is characterized by disseminated deep-red erythematous areas and pustules, which may merge to extensive lakes of pus. In contrast, there are two localized variants termed palmoplantar pustulosis and acrodermatitis continua suppurativa (Fig. 1H, depicting onychodystrophy in the latter condition). Despite its wider clinical spectrum, pustular psoriasis is quite rare as compared with nonpustular forms. Both pustular and the more common vulgar forms may progress to psoriatic erythroderma affecting the entire body surface (Fig. 1C). Psoriatic arthritis is an extracutaneous manifestation that affects at least 5 percent and perhaps as many as 20 percent of patients with psoriasis (Fig. 1I).25,26 The nails are affected in the majority of patients with psoriatic arthritis, but nail involvement can be seen in all types of psoriasis. The fingernails are more frequently affected than are toenails (on average, in 50 percent of patients as compared with 35 percent), and lesions range from pits and yel- www.nejm.org may 5 , 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. medical progress A B C D F I E H G J n engl j med 352;18 K www.nejm.org may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1901 The new england journal lowish discoloration (Fig. 1J and 1K) to severe onychodystrophy, a typical complication of acrodermatitis continua suppurativa (Fig. 1H).25,26 For many patients, the symptoms of psoriasis improve in the summer and worsen in the winter, reflecting the well-established notion that the course of the disease is influenced by various environmental factors. Physical trauma may trigger psoriatic lesions at sites of injury (Koebner’s phenomenon), possibly through the release of proinflammatory cytokines, the unmasking of autoantigens, or both.27 In fact, some treatments for psoriasis that have proinflammatory potential (e.g., anthralin and phototherapy) appear to trigger psoriasis if applied too aggressively — for example, in high initial doses. Molecular mechanisms underlying drug-induced flares of psoriasis are incompletely understood. Mechanisms for certain medications are partially delineated. For example, beta-adrenergic blockers may induce epidermal hyperproliferation associated with a decrease of intraepidermal cyclic AMP; lithium may elevate proinflammatory cytokines, thereby stimulating cutaneous leukocyte recruitment; and chloroquine blocks epidermal transglutaminase, an enzyme that is pivotally involved in the terminal differentiation of keratinocytes.28 Infections, particularly streptococcal infections of the upper respiratory tract, have long been recognized as triggers of psoriasis.24 In addition, exacerbation or even initial manifestation of psoriasis has been observed in patients infected with the human immunodeficiency virus (HIV). However, because of clinical similarities, psoriasis in patients with HIV infection may be misinterpreted as seborrheic eczema.29 Psoriatic skin exhibits pathological changes in most, if not all, cutaneous cell types. The typical erythematosquamous plaque contains histopathological hallmark features that include hyperproliferation of epidermal keratinocytes and hyperkeratosis, as well as infiltration of immunocytes along with angiogenesis, with resultant typical thickening and scaling of the erythematous skin. Mitotic activity of basal keratinocytes is increased by as much as a factor of 50 in psoriatic skin, so keratinocytes need only 3 to 5 days in order to move from the basal layer to the cornified layer (instead of the normal 28 to 30 days). This dramatically shortened maturation time is accompanied by altered differentiation, reflected by the focal absence of the granular layer of the epidermis and parakeratosis, or nuclei still present in the thickened cornified layer (Fig. 2A through 2D). 1902 n engl j med 352;18 of medicine Figure 2 (facing page). Complex Pathological Tissue Alterations in Psoriatic Skin. As compared with normal skin (Panel A), the epidermis in psoriatic skin (Panel B) is characterized by dramatic histopathological alterations, including profound acanthosis (thickening of the viable cell layers) with elongation of epidermal rete ridges (arrowheads), marked hyperkeratosis (thickening of the cornified layer), loss of the granular layer, and parakeratosis (nuclei in the stratum corneum). In addition, dermal blood vessels are increased in number and size (by both angiogenesis and dilatation); they are contorted and reach up to locations directly underneath the epidermis (arrows). Finally, a mixed leukocytic infiltrate is seen in both dermis and epidermis. As a histopathological hallmark of psoriatic lesions, neutrophilic granulocytes transmigrate through the epidermis (Panel C, arrow) and form the telltale Munro microabscesses underneath the stratum corneum (Panel C, arrowhead). As the lesions progress, these microabscesses are transported to the upper layers of the stratum corneum, where they slough off (Panel D, arrow). (Panels A through D show staining with hematoxylin and eosin.) Focal expression of intercellular adhesion molecule 1 (ICAM-1) in psoriatic epidermis (Panel E) indicates the activation of keratinocytes. Immunostaining of CD3, a T-cell receptor–associated antigen, shows that abundant T lymphocytes are present in psoriatic skin within both the dermis and the epidermis (Panel F). The integrin aE(CD103)b7, an adhesion receptor that binds to epidermal E-cadherin, is expressed almost exclusively by intraepidermal T cells (Panel G). (Panels E through G are highlighted with an immunoperoxidase stain.) It is thought that ICAM-1, the aE(CD103)b7 integrin, and other adhesion receptors contribute to the recruitment of pathogenic lymphocytes to psoriatic skin. Psoriatic epidermis demonstrates aberrant expression of antigens associated with hyperproliferation, such as the heterodimer keratin 6–keratin 16 and heat-shock proteins. In addition, induced expression of MHC class II antigens and intercellular adhesion molecule 1 (ICAM-1) is observed.4,30 These molecules are involved in interactions with lymphocytes such as adhesion or antigen recognition (Fig. 2E). Moreover, vascular endothelial cells are primed to take part in angiogenesis in psoriasis, and blood vessels that are associated with psoriatic lesions become dilated and contorted, reaching directly beneath the epidermis in the dermal papillar regions (Fig. 2B). The involved vascular endothelial cells express increased levels of ICAM-1 (CD54), E-selectin (CD62E), vascular-cell adhesion molecule 1 (CD106), and MHC class II antigens, indicating activation.31 Then a mixed inflammatory infiltrate that contains activated CD3+ T lymphocytes within both the dermis and epidermis is evident (Fig. 2F),32 within which CD4+ T cells are www.nejm.org may 5 , 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. medical progress A B D C E I F G J K distributed randomly. In contrast, the majority of CD8+ T cells, some of which express epithelial-specific adhesion receptors (Fig. 2G),33 reside preferentially within the epidermis.34 Many T cells within psoriatic skin exhibit an acn engl j med 352;18 tivated phenotype characterized by increased expression of costimulatory molecules such as CD2, adhesion molecules including leukocyte-function–associated antigen 1 (LFA-1) and cutaneous lymphocyte-associated antigen (CLA), or the high- www.nejm.org may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1903 The new england journal affinity interleukin-2 receptor. Neutrophils localize to the dermis, migrate focally into the epidermis, and form Munro microabscesses, which become translocated upward within the epidermal stratum corneum (Fig. 2C and 2D).35 In addition, psoriatic skin contains an increased number of dermal mast cells and dendritic cells.36 immunopathogenesis Psoriasis is an instructive model for studying interactions of immigrating immunocytes with resident epithelial and mesenchymal cells. This disease vividly highlights the pathogenic importance of T cells and simultaneously illustrates how advances in our understanding of molecular immune mechanisms can be translated into innovative therapies. Although many factors that contribute to the generation of psoriatic lesions remain obscure, compelling circumstantial and experimental evidence suggests a primary T-lymphocyte–based immunopathogenesis (Fig. 3).30 The response of psoriasis to treatment with compounds that act on lymphocytes, such as cyclosporine, first described for use in psoriasis in 1979, is an early example.38 More recently, other compounds specifically targeting T-cell functions were found to alleviate psoriasis. These include interleukin-2 fused to truncated diphtheria toxin (DAB389IL-2)39 and antibodies directed against CD2,40 CD11a,41,42 or, in some cases, CD4.43,44 In addition, there is a possible linkage of the psoriasis-susceptibility gene PSORS2 with a gene involved in the regulation of interleukin-2.17 Furthermore, psoriasis may not recur after bone marrow transplantation (performed in order to treat disorders unrelated to psoriasis) from healthy donors45 or may develop for the first time after bone marrow transplantation from a donor with psoriasis.46 The aforementioned association of psoriasis with certain MHC alleles — such as HLA-B13, HLA-B17, HLA-Bw57, and HLA-Cw6 — also suggests a pathogenic role of T cells.4 Indeed, some investigators have reported that there is a restricted use of T-cell receptor variable genes within psoriatic lesions, a finding that implies antigen-specific T-cell responses.47 Although the pathogenic relevance of this oligoclonal T-cell expansion is not entirely clear,48 it is possible that the failure to demonstrate oligoclonality in some cases of psoriasis is due, at least in part, to the colonization of psoriatic lesions by bacteria that produce superantigen, triggering the T-cell receptor without using the var- 1904 n engl j med 352;18 of medicine Figure 3 (facing page). Putative T-Cell Responses in the Pathogenesis of a Psoriatic Lesion. To generate a cutaneous T-cell response, antigen-presenting cells (Langerhans’ cells in the epidermis) take up and process autoantigens and migrate to the regional lymph nodes. There, they come in contact with naive T lymphocytes (CD45RA+). Within an immunologic synapse (inset), molecular interactions result in T-cell activation.37 According to the putative theory, antigen-presenting cells present processed antigen bound to major-histocompatibility-complex (MHC) molecules to the T-cell receptor (TCR). MHC class II molecules present antigen to CD4+ T cells, whereas MHC class I molecules present antigen to CD8+ T cells. Additional signals are transmitted through interactions of costimulatory molecules with their ligands such as CD2 with CD58, CD28 with either CD80 or CD86, or both. In addition, adhesive interactions (e.g., by integrins and their immunoglobulin superfamily ligands) also stabilize the immunologic synapse and transmit additional signals. Following the activating signals, T cells differentiate into CD45RO+ memory T cells and express skin-homing receptor cutaneous lymphocyte-associated antigen (CLA). Once activated, T cells (CD45RO+ and CLA+) reenter the circulation and preferentially extravasate at sites of cutaneous inflammation. In the skin, on encountering the respective antigen, T cells exert their effector functions, which include the secretion of proinflammatory cytokines. Psoriasis is characterized by a chronically persisting response in effector T cells. ICAM-1 denotes intercellular adhesion molecule 1. iable antigen-recognition sites.49 The permissive role of bacterial superantigens in the pathogenesis of psoriasis is well established.49 In addition, sequence similarities between streptococcal M peptides and human keratins, such as keratin 17, led to the hypothesis that keratinocyte proteins function as autoantigens in psoriasis.50 The generation of psoriasis-like skin lesions has been described in animal models, by a process based on T-cell dysregulation without prior epithelial abnormalities.51,52 In addition, the role of T lymphocytes as key effector cells is strongly supported by work from various groups in xenotransplantation models. Injection of activated T lymphocytes from patients with psoriasis into unaffected skin transplanted from the same patients onto SCID (severe combined immunodeficiency) mice resulted in the generation of psoriatic lesions in the animals.53-55 Although additional rodent models support the prominent role of T lymphocytes, it is also clear that these T lymphocytes can trigger the disease only in a susceptible environment, since these results are obtained with skin from psoriatic, but not from healthy, donors.56 www.nejm.org may 5 , 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. medical progress Psoriatic plaque Release of chemokines and cytokines Lymphocyte Neutrophils Uninvolved skin Epidermis Macrophages Dermis Migration to lymph nodes Proliferation of lymphocytes Activation of lymphocytes CD80 Putative antigen or presented by CD86 MHC MHC CD3 CD58 Recirculation of lymphocytes ICAM-1 LFA-1 CD4 CD2 TCR CD28 T cell n engl j med 352;18 www.nejm.org may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1905 The new england journal cytokines, chemokines, and adhesion molecules Psoriasis may serve as a model of a disease that clearly shows the central role of cytokines and chemokines and the functional interaction of these proteins with adhesion molecules in the recruitment of tissue-specific lymphocytes.57,58 Cytokine dysregulation may explain, at least in part, the complex tissue alterations in psoriasis (Fig. 4). Proinflammatory cytokines59-62 induce the expression of adhesion molecules on endothelial cells and keratinocytes, allowing them to interact with leukocytes. This results in leukocyte extravasation at the site of inflammation, along with migration through the cutaneous matrix toward the epidermis.63,64 Numerous studies have identified tumor necrosis factor a (TNF-a) as a particularly relevant cytokine regulating this complex inflammatory cascade. Its key role is underlined by the therapeutic efficacy of compounds that interfere with TNF-a functions.41,65,66 Psoriatic skin is further characterized by increased angioneogenesis in a milieu rich in proangiogenic factors.31,67 Complementary to the up-regulation of proinflammatory cytokines, reduced levels of the antiinflammatory cytokine interleukin-10, along with the relative dominance of type 1 helper T (Th1) cytokines, such as interferon-g and interleukin-2, add to a milieu with mediator imbalance.68-70 The exact mechanisms by which these cytokines regulate the microenvironment that influences psoriasis need further clarification. Recent evidence strongly suggests that chemokines are pivotal for the trafficking and compartmentalization of leukocytes in the psoriatic disease process (Fig. 4).58,71 The effector-T-cell population that is putatively the most relevant in psoriasis is characterized by the expression of skin-homing receptor CLA and the CC chemokine receptor 4 (CCR4).72 Other chemokines that are implicated in the recruitment of T cells into psoriatic plaques include CCL20 (macrophage inflammatory protein 3a, or MIP-3a),58 CCL27 (cutaneous T-cell–attracting chemokine, or CTACK),71 monokine induced by interferon-g (MIG),73 or RANTES (regulated on activation, normal T-cell expressed and secreted), and monocyte chemotactic protein 1 (MCP-1). It is thought that neutrophils, another leukocyte population abundantly present in psoriatic infiltrates, are recruited by the neutrophil-attracting chemokine interleukin-8 (CXCL8). However, this pathway is probably not the exclusive means of 1906 n engl j med 352;18 of medicine Figure 4 (facing page). The Cytokine and Chemokine Network. The transition from normal skin to the full-fledged psoriatic lesion is orchestrated by complex interactions of various cytokines and chemokines. Proinflammatory cytokines are thought to account for many of the histopathological changes seen in psoriatic skin. For example, interferon-g and tumor necrosis factor a (TNF-a) can stimulate the expression of major-histocompatibilitycomplex (MHC) class II molecules and intercellular adhesion molecule 1 (ICAM-1). Vascular endothelial growth factor (VEGF) and TNF-a stimulate angiogenesis. At the same time, interleukin-1 activates mast cells, granulocyte–macrophage colony-stimulating factor (GM-CSF) activates neutrophils, nerve growth factor (NGF) stimulates the growth of cutaneous nerves, and interleukin-6 and transforming growth factor a (TGF-a) promote keratinocyte proliferation. TNF-a, in particular, appears to affect the functions of many different cell types in psoriatic skin. Thymus- and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC) are expressed by the cutaneous vasculature and contribute to the recruitment of CCR4+ T cells. Cutaneous T-cell–attracting chemokine (CTACK) contributes to epidermal recruitment of T cells expressing its receptor, CCR10. In addition, macrophage inflammatory protein 3a (MIP-3a) colocalizes in psoriatic epidermis with epidermal T cells expressing its receptor, CCR6, and can be induced on keratinocytes by proinflammatory cytokines, such as TNF-a. Another T-cell–attracting chemokine, monokine induced by interferon-g (MIG), is expressed by endothelial cells and macrophages directly underneath the hyperplastic psoriatic epidermis. Since MIG can be induced by T-cell–derived interferon-g, there is a microenvironmental T-cell–associated inflammationboosting loop. This process may be augmented by RANTES (regulated on activation, normal T-cell expressed and secreted) and monocyte chemotactic protein 1 (MCP-1), both of which also attract mast cells to psoriatic skin. Within psoriatic scales, there is a high content of interleukin-8 and growth-related cytokine a (GRO-a), both of which are neutrophil-attracting chemokines that contribute to the formation of Munro microabscesses, a hallmark of psoriatic epidermis. Keratinocyte hyperproliferation in psoriatic skin also appears to be induced, at least in part, by interleukin-8 and GRO-a. neutrophil recruitment, since an interleukin-8– blocking monoclonal antibody had only modest efficacy in a clinical study.74 In addition to the mediators involved in leukocyte recruitment and activation, substances such as neuropeptides appear to be involved in the pathogenesis of psoriasis. These include substance P and nerve growth factor, along with its receptor, the p75 neurotrophin receptor, and tyrosine kinase A.75,76 That neuropathogenic mechanisms contribute to www.nejm.org may 5 , 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. medical progress Neutrophils Increased proliferation and turnover of keratinocytes Keratinocytes T lymphocytes Macrophage TARC MDC MCP-1 RANTES Interleukin-8 GRO-a Interleukin-1 Cutaneous nerve VEGF GM-CSF MIP-3a MCP-1 RANTES Mast cell Interleukin-6 TGF-a Interferon-g Endothelial cell a MIG n engl j med 352;18 www.nejm.org may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1907 The new england journal the development of psoriasis is further suggested by the increase in terminal cutaneous nerves within psoriatic lesions. Finally, clinical observations, such as the symmetric distribution pattern of psoriatic lesions and the resolution of psoriasis at sites of administration of local anesthesia, are currently interpreted as evidence of the intimate involvement of the nervous system in the pathogenesis of psoriasis. As in other inflammatory disorders, leukocyte recruitment to psoriatic skin occurs in consecutive steps mediated by complex interactions of cytokines, chemokines, and adhesion receptors.77,78 Although psoriasis-specific steps have not been identified, this disease has served as a model for indepth investigations of several adhesion-molecule interactions that are of general importance in the generation of inflammatory reactions.41,42 The first step of leukocyte recruitment is the transition from free flow in the vascular lumen to a rolling motion along the endothelium of the vessel wall, mediated primarily by a family of adhesion molecules called selectins.77-80 Activated endothelial cells express P-selectin (CD62P) and E-selectin (CD62E).81 The pivotal role of selectins in leukocyte rolling has been confirmed in many experimental approaches that interfere with their adhesive interactions.81 Selectin functions overlap to a considerable extent, as can be concluded from the efficacy of selectin-blocking strategies. A monoclonal antibody that was specifically directed against E-selectin failed to alleviate psoriasis in a recent clinical trial, whereas less selective compounds, such as efomycine M, which inhibits both E-selectin and P-selectin, showed significant anti-psoriatic efficacy in animal models.82,83 Endothelial cells express E-selectin, which can interact with specific ligands expressed by T cells. These ligands are transmembrane glycoproteins bearing a special carbohydrate moiety, called sialylLewisX, displayed on cell-surface proteins.81 T lymphocytes localizing to the skin express the sialylLewisX –bearing CLA,84 which is thought to confer the tissue selectivity of these cells, at least in part.85 Once rolling leukocytes are subject to activation by chemokines, they attach firmly to the endothelium through the interactions between b2 integrins (e.g., LFA-1, or aL b 2 integrin) and adhesion molecules of the immunoglobulin superfamily, such as ICAM-1 (CD54). Additional interactions occur between b1 integrins and their ligands, such as a4b1 integrin and vascular-cell adhesion molecule 1. The importance of LFA-1 in T-cell recruitment into 1908 n engl j med 352;18 of medicine the skin is highlighted by the antipsoriatic efficacy of efalizumab, a new monoclonal antibody directed against LFA-1.41,42 In contrast to the relatively well understood process of lymphocyte extravasation, little is known about the subsequent migration through the cutaneous extracellular matrix and the processes determining the ultimate localization and arrest of lymphocytes within the epidermal compartment. The epidermis of psoriatic skin is characterized by induced expression of ICAM-1 (Fig. 2E).63,86 ICAM-1, induced by proinflammatory cytokines, enables activated lymphocytes to attach through their surface molecules, such as LFA-1.42 In addition, other adhesion molecules may contribute to epidermal T-cell localization.78 For example, the aE(CD103)b7 integrin (Fig. 2G), which binds to epidermal E-cadherin,87 has been implicated in epidermal recruitment of CD8+ T cells in psoriatic lesions.33,88 However, it is also possible that lymphocytes are transported passively to the upper epidermal layers owing to the accelerated upward migration of keratinocytes in psoriasis. Overall, given the central role of T lymphocytes in the pathogenesis of psoriasis, agents that would influence the function or recruitment of leukocytes are appealing therapeutic compounds.89,90 therapy Most accepted treatments for psoriasis have been developed empirically or were found by chance. However, recent insights into the immunopathogenesis of psoriasis have further elucidated the mode of action of some accepted compounds91,92 and have provided new treatment strategies.93,94 The severity of the disease usually determines the therapeutic approach. Approximately 70 to 80 percent of all patients with psoriasis can be treated adequately with use of topical therapy. Mainly for practical reasons, the vitamin D3 analogues (calcipotriol and tacalcitol) and the topical retinoid tazarotene — all of which affect keratinocyte functions and the immune response — are in wider use than is either anthralin or coal tar. Since most of the compounds that have been mentioned may irritate delicate areas of skin, topical corticosteroids are used in combination with those compounds, particularly in intertriginous areas. In cases of moderate-to-severe psoriasis (e.g., affecting large surface areas), the use of phototherapy, systemic drugs, or both must be considered. www.nejm.org may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. medical progress Among the established regimens, various therapeutic methods may have distinct modes of action. For example, fumarates and cyclosporine are primarily immunosuppressive agents, whereas retinoids and methotrexate also target keratinocyte functions. Rational combination treatments target inflammation as well as epidermal alterations and may provide improved efficacy and safety. Thus, combinations of topical vitamin D3 analogues with phototherapy or systemic retinoids plus psoralen and ultraviolet A phototherapy (RePUVA) are well-established treatment regimens for psoriasis. Psoriasis in children, in pregnant women, or in patients with the acquired immunodeficiency syndrome may provide considerable therapeutic challenges, arguably best handled by consultation with a specialist. Likewise, severe nail involvement or pustular psoriasis should be the province of the specialist. Although most established treatment regimens are reasonably effective as short-term therapy for psoriasis, extended disease control is difficult to achieve because the safety profile of most therapeutic agents limits their long-term use.95 Another unmet medical need is for agents that can be applied easily, since application of various currently available agents is difficult and thus compliance may be problematic. More convenient preparations improve adherence to recommended regimens.96 Patients with severe psoriasis often become frustrated with the management of their disease and the perceived ineffectiveness of the therapies prescribed.97 Studies have indicated that the impairment of the quality of life caused by psoriasis equals or even exceeds that due to other major illnesses such as diabetes, rheumatoid arthritis, and cancer.4,98 As already noted, recent advances in psoriasis research have provided a sound platform for the rational design of new biologic agents and biologicimmune-response modifiers that specifically target key mechanisms of the pathogenesis of psoriasis.41 Three of these agents — alefacept, efalizumab, and etanercept — are currently approved by the Food and Drug Administration (FDA) for the treatment of psoriasis; several others (e.g., infliximab) are in the final phase of clinical development. The most promising compounds are monoclonal antibodies, cytokines, and fusion proteins. Three fundamental modes of action are being explored: decreasing the number of pathogenic T cells, blocking T-cell migration and adhesion, and antagonizing effector cytokines (Fig. 5). n engl j med 352;18 A Inhibition of T-cell activation Antigenpresenting cell B T cell TCR/CD3 CD4 Adhesion molecules Release of chemokines TNF-a Th2 Th1 Th2 Figure 5. New Pathogenesis-Oriented Therapeutic Principles. Five general therapeutic principles target the key pathogenic mechanisms of psoriasis. The first involves inhibition of T-cell activation through inhibition of molecules involved in the formation of the immunologic synapse (Panel A). The second principle is depletion of pathogenic T cells (Panel B). This has been achieved by targeting molecules expressed specifically by activated T cells, such as the high-affinity interleukin-2 receptor or CD4. The third approach involves inhibition of leukocyte recruitment to the inflamed skin — for example, by inhibiting key adhesion molecules such as selectins or certain integrins (Panel C). A prominent example is the use of efalizumab, a monoclonal antibody that interferes with adhesion mediated by leukocyte-function–associated antigen 1 (LFA-1). The fourth principle is functional inhibition of key inflammatory cytokines (Panel D). Perhaps the most important example is tumor necrosis factor a (TNF-a), the functions of which are targeted by several biologic agents, the monoclonal antibodies infliximab and adalimumab, and the fusion proteins etanercept and onercept. Finally, it is possible to induce an immune deviation to shift the cytokine milieu dominated by type 1 helper T (Th1) cells to a milieu weighted with type 2 helper T (Th2) cells, thus alleviating psoriasis. This has been demonstrated in principle for interleukin-10 and interleukin-4 (Panel E). www.nejm.org may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1909 The new england journal As demonstrated by antibody-mediated targeting of CD4 on helper T cells43,44,99 or by targeting of the T-cell–expressed interleukin-2 receptor with an interleukin-2–diphtheria toxin,39 psoriasis can be alleviated by decreasing the number of pathogenic T cells. The first biologic agent approved by the FDA for treating psoriasis was alefacept, a fusion protein in which the binding site of leukocytefunction–associated antigen 3 (LFA-3) and the human IgG Fc portion are combined. Alefacept binds to CD2 on activated T cells, thus impairing costimulatory signals delivered by LFA-3 and (possibly the best explanation for the long-lasting effect in the subgroup of patients who respond to the drug) inducing apoptosis in circulating memory T cells.40 Interruption of the molecular cascade resulting in cutaneous recruitment of pathogenic leukocytes has been achieved by efalizumab, a humanized monoclonal antibody that is directed against an extracellular epitope of the LFA-1 a chain, which was approved by the FDA for the treatment of psoriasis in October 2003.42 Other substances, which include small-molecule compounds, are currently under development.78 A key cytokine in psoriasis (and in other inflammatory diseases) is TNF-a, which can be function- of medicine ally inhibited by the chimeric antibody infliximab or by the recombinant human TNF-receptor fusion protein etanercept. Both agents competitively inhibit interactions of TNF-a with cell-surface receptors and show convincing efficacy in treating psoriasis.65,66 Shifting the immunologic microenvironment in psoriatic skin, dominated by Th1-type cytokines through substitution of type 2 helper T-cell–type cytokines, such as interleukin-1069 and interleukin-4,70 has been reported as effective in some cases of psoriasis. Numerous other biologic agents and immuneresponse modifiers are under development; all of them use at least one of the mechanisms mentioned above.100 As a class, these compounds have the potential to handle some of the unmet medical needs in the treatment of psoriasis. Supported by a Rudolf Virchow Award and a research grant (Scho 565/5-1) from the Deutsche Forschungsgemeinschaft (to Dr. Schön). Dr. Schön reports having received lecture fees from Serono and grant support from 3M Pharmaceuticals. Dr. Boehncke reports having received consulting and lecture fees from Serono, Biogen, and Wyeth and lecture fees from Schering AG. We are indebted to Professor U. Mrowietz (University of Kiel, Kiel, Germany) for helpful discussions and critical proofreading of the manuscript and to Professor C.E.M. Griffiths (University of Manchester, Manchester, United Kingdom) for a helpful suggestion. refer enc es 1. Lomholt G. Prevalence of skin diseases in a population; a census study from the Faroe Islands. Dan Med Bull 1964;11:1-7. 2. Farber EM, Nall ML. Epidemiology: natural history and genetics. In: Roenigk HH, Maibach HI, eds. Psoriasis. New York: Marcel Dekker, 1998:107-58. 3. Kenney JA. Psoriasis in the American black. In: Farber EM, Cox AJ, eds. Psoriasis: proceedings of the International Symposium, Stanford University. Stanford, Calif.: Stanford University Press, 1971:49-52. 4. Christophers E. Psoriasis — epidemiology and clinical spectrum. Clin Exp Dermatol 2001;26:314-20. 5. Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985;13:450-6. 6. Watson W, Cann HM, Farber EM, Nall ML. The genetics of psoriasis. Arch Dermatol 1972;105:197-207. 7. Farber EM, Nall ML, Watson W. Natural history of psoriasis in 61 twin pairs. Arch Dermatol 1974;109:207-11. 8. Brandrup F, Holm N, Grunnet N, Henningsen K, Hansen HE. Psoriasis in monozygotic twins: variations in expression in individuals with identical genetic constitution. Acta Derm Venereol 1982;62:229-36. 9. Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical re- 1910 view of 1262 cases. Pediatr Dermatol 2001; 18:188-98. 10. Altmüller J, Palmer LJ, Fischer G, Scherb H, Wjst M. Genomewide scans of complex human diseases: true linkage is hard to find. Am J Hum Genet 2001;69:93650. [Erratum, Am J Hum Genet 2001;69: 1413.] 11. Davidson A, Diamond B. Autoimmune diseases. N Engl J Med 2001;345:340-50. 12. Trembath RC, Clough RL, Rosbotham JL, et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet 1997;6:813-20. 13. Burden AD, Javed S, Bailey M, Hodgins M, Connor M, Tillman D. Genetics of psoriasis: paternal inheritance and a locus on chromosome 6p. J Invest Dermatol 1998; 110:958-60. 14. Mallon E, Bunce M, Wojnarowska F, Welsh K. HLA-CW*0602 is a susceptibility factor in type I psoriasis, and evidence Ala73 is increased in male type I psoriatics. J Invest Dermatol 1997;109:183-6. 15. Asumalahti K, Veal C, Laitinen T, et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum Mol Genet 2002;11:589-97. 16. Allen MH, Veal C, Faassen A, et al. n engl j med 352;18 www.nejm.org A non-HLA gene within the MHC in psoriasis. Lancet 1999;353:1589-90. 17. Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 1994;264:1141-5. 18. Matthews D, Fry L, Powles A, et al. Evidence that a locus for familial psoriasis maps to chromosome 4q. Nat Genet 1996; 14:231-3. 19. Capon F, Novelli G, Semprini S, et al. Searching for psoriasis susceptibility genes in Italy: genome scan and evidence for a new locus on chromosome 1. J Invest Dermatol 1999;112:32-5. 20. Enlund F, Samuelsson L, Enerback C, et al. Psoriasis susceptibility locus in chromosome region 3q21 identified in patients from southwest Sweden. Eur J Hum Genet 1999;7:783-90. 21. Lee YA, Ruschendorf F, Windemuth C, et al. Genomewide scan in German families reveals evidence for a novel psoriasis-susceptibility locus on chromosome 19p13. Am J Hum Genet 2000;67:1020-4. 22. Veal CD, Clough RL, Barber RC, et al. Identification of a novel psoriasis susceptibility locus at 1p and evidence of epistasis between PSORS1 and candidate loci. J Med Genet 2001;38:7-13. 23. Helms C, Cao L, Krueger JG, et al. A putative RUNX1 binding site variant be- may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. medical progress tween SLC9A3R1 and NAT9 is associated with susceptibility to psoriasis. Nat Genet 2003;35:349-56. 24. Owen CM, Chalmers RJ, O’Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev 2000;2: CD001976. 25. Lavaroni G, Kokelj F, Pauluzzi P, Trevisan G. The nails in psoriatic arthritis. Acta Derm Venereol Suppl (Stockh) 1994;186:113. 26. Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 2003;4:441-7. 27. Eyre RW, Krueger GG. Response to injury of skin involved and uninvolved with psoriasis and its relation to disease activity: Koebner and ‘reverse’ Koebner. Br J Dermatol 1982;106:153-9. 28. Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis: recognition and management. Am J Clin Dermatol 2000;1: 159-65. 29. Mallon E. Retroviruses and psoriasis. Curr Opin Infect Dis 2000;13:103-7. 30. Christophers E. The immunopathology of psoriasis. Int Arch Allergy Immunol 1996; 110:199-206. 31. Detmar M, Brown LF, Claffey KP, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994; 180:1141-6. 32. Schlaak JF, Buslau M, Jochum W, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol 1994; 102:145-9. 33. Pauls K, Schön M, Kubitza RC, et al. Role of integrin aE(CD103)b7 for tissuespecific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol 2001;117: 569-75. 34. Ovigne J-M, Baker BS, Davison SC, Powles AV, Fry L. Epidermal CD8+ T cells reactive with group A streptococcal antigens in chronic plaque psoriasis. Exp Dermatol 2002;11:357-64. 35. Pinkus H, Mehregan AH. The primary histologic lesion of seborrheic dermatitis and psoriasis. J Invest Dermatol 1966;46: 109-16. 36. Rothe MJ, Nowak M, Kerdel FA. The mast cell in health and disease. J Am Acad Dermatol 1990;23:615-24. 37. Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science 1999;285:221-7. 38. Mueller W, Herrmann B. Cyclosporin A for psoriasis. N Engl J Med 1979;301:555. 39. Gottlieb JL, Gilleaudeau P, Johnson R, et al. Response of psoriasis to a lymphocyteselective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med 1995;1:442-7. 40. Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001;345:248-55. 41. Kupper TS. Immunologic targets in psoriasis. N Engl J Med 2003;349:1987-90. 42. Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med 2003;349:2004-13. 43. Prinz J, Braun-Falco O, Meurer M, et al. Chimaeric CD4 monoclonal antibody in treatment of generalized pustular psoriasis. Lancet 1991;338:320-1. 44. Nicolas JF, Chamchick N, Thivolet J, Wijdenes J, Morel P, Revillard JP. CD4 antibody treatment of severe psoriasis. Lancet 1991;338:321. 45. Eedy DJ, Burrows D, Bridges JM, Jones FGC. Clearance of severe psoriasis after allogenic bone marrow transplantation. BMJ 1990;300:908. 46. Gardembas-Pain M, Ifrah N, Foussard C, Boasson M, Saint Andre JP, Verret JL. Psoriasis after allogeneic bone marrow transplantation. Arch Dermatol 1990;126:1523. 47. Bour H, Puisieux I, Kourilsky P, Favrot M, Musette P, Nicolas JF. T-cell repertoire analysis in chronic plaque psoriasis suggests an antigen-specific immune response. Hum Immunol 1999;60:665-76. 48. Vekony MA, Holder JE, Lee AJ, Horrocks C, Eperon IC, Camp RD. Selective amplifications of T-cell receptor variable region species is demonstrable but not essential in early lesions of psoriasis vulgaris: analysis by anchored polymerase chain reaction and hypervariable region size spectratyping. J Invest Dermatol 1997;109:5-13. 49. Boehncke W-H. Psoriasis and bacterial superantigens — formal or causal correlation? Trends Microbiol 1996;4:485-9. 50. Gudmundsdottir AS, Sigmundsdottir H, Sigurgeirsson B, Good MF, Valdimarsson H, Jonsdottir I. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis? Clin Exp Immunol 1999; 117:580-6. 51. Schön MP, Detmar M, Parker CM. Murine psoriasis-like disorder induced by naive CD4+ T-cells. Nat Med 1997;3:183-8. 52. Breban M, Fernandez-Sueiro JL, Richardson JA, et al. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol 1996;156:794-803. 53. Boehncke W-H, Dressel D, Zollner TM, Kaufmann R. Pulling the trigger on psoriasis. Nature 1996;379:777. 54. Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest 1996;98:1878-87. 55. Schön MP. Animal models of psoriasis — what can we learn from them? J Invest Dermatol 1999;112:405-10. 56. Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med 2004;199:731-6. 57. Nickoloff BJ. The cytokine network of psoriasis. Arch Dermatol 1991;127:871-84. n engl j med 352;18 www.nejm.org 58. Schön MP, Ruzicka T. Psoriasis: the plot thickens . . . . Nat Immunol 2001;2:91. 59. Murphy JE, Robert C, Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol 2000;114:602-8. 60. Barker JNWN, Karabin GD, Stoof TJ, Sarma VJ, Dixit VM, Nickoloff BJ. Detection of interferon-gamma mRNA in psoriatic epidermis by polymerase chain reaction. J Dermatol Sci 1991;2:106-11. 61. Neuner P, Urbanski A, Trautinger F, et al. Increased IL-6 production by monocytes and keratinocytes in patients with psoriasis. J Invest Dermatol 1991;97:27-33. 62. Elder JT, Fisher GJ, Lindquist PB, et al. Overexpression of transforming growth factor a in psoriatic epidermis. Science 1989; 243:811-4. 63. Griffiths CEM, Voorhees JJ, Nickoloff BJ. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol 1989; 20:617-29. 64. Barker JNWN, Sarma V, Mitra RS, Dixit VM, Nickoloff BJ. Marked synergism between tumor necrosis factor-alpha and interferon-gamma in regulation of keratinocytederived adhesion molecules and chemotactic factors. J Clin Invest 1990;85:605-8. 65. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349: 2014-22. 66. Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001;357:1842-7. 67. Kuroda K, Sapadin A, Shoji T, Fleischmajer R, Lebwohl M. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol 2001;116: 713-20. 68. Michel G, Mirmohammadsadegh A, Olasz E, et al. Demonstration and functional analysis of IL-10 receptors in human epidermal cells: decreased expression in psoriatic skin, down-modulation by IL-8, and upregulation by an antipsoriatic glucocorticosteroid in normal cultured keratinocytes. J Immunol 1997;159:6291-7. 69. Asadullah K, Sterry W, Stephanek K, et al. IL-10 is a key cytokine in psoriasis: proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest 1998;101: 783-94. 70. Ghoreschi K, Thomas P, Breit S, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med 2003;9:40-6. 71. Homey B, Alenius H, Müller A, et al. CCL27-CCR10 interactions regulate T cellmediated skin inflammation. Nat Med 2002;8:157-65. 72. Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved. 1911 medical progress recognition by cutaneous but not intestinal memory T cells. Nature 1999;400:776-80. 73. Goebeler M, Toksoy A, Spandau U, Engelhardt E, Bröcker E-B, Gillitzer R. The C-X-C chemokine Mig is highly expressed in the papillae of psoriatic lesions. J Pathol 1998;184:89-95. 74. Homey B. Chemokines and chemokine receptors as targets in the therapy of psoriasis. Curr Drug Targets Inflamm Allergy 2004;3:169-74. 75. Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M, Luger TA. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol 2003;139:1479-88. 76. Raychaudhuri SP, Raychaudhuri SK. Role of NGF and neurogenic inflammation in the pathogenesis of psoriasis. Prog Brain Res 2004;146:433-7. 77. Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med 1999;341:1817-28. 78. Schön MP, Zollner TM, Boehncke WH. The molecular basis of lymphocyte recruitment to the skin: clues for pathogenesis and selective therapies of inflammatory disorders. J Invest Dermatol 2003;121:951-62. 79. Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996;272:60-6. 80. von Andrian UH, Mackay CR. T-cell function and migration: two sides of the same coin. N Engl J Med 2000;343:1020-34. 81. Ley K. Functions of selectins. Results Probl Cell Differ 2001;33:177-200. 82. Bhushan M, Bleiker TO, Ballsdon AE, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. Br J Dermatol 2002;146:824-31. 83. Schön MP, Krahn T, Schön M, et al. Efomycine M, a new specific inhibitor of selec- 1912 tin, impairs leukocyte adhesion and alleviates cutaneous inflammation. Nat Med 2002; 8:366-72. [Erratum, Nat Med 2002;8:639.] 84. Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 1997;389:978-81. 85. Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans: preferential expression of the HECA-425 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol 1990;136:1053-68. 86. Dustin ML, Singer KH, Tuck DT, Springer TA. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med 1988;167:1323-40. 87. Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 1994;372: 190-3. 88. Rottman JB, Smith TL, Ganley KG, Kikuchi T, Krueger JG. Potential role of the chemokine receptors CXCR3, CCR4, and the integrin alphaEbeta7 in the pathogenesis of psoriasis vulgaris. Lab Invest 2001;81: 335-47. 89. Schön MP, Drewniok C, Boehncke W-H. Targeting selectin functions in the therapy of psoriasis. Curr Drug Targets Inflamm Allergy 2004;3:163-8. 90. Boehncke W-H, Schön MP. Interfering with leukocyte rolling — a promising therapeutic approach in inflammatory skin disorders? Trends Pharmacol Sci 2003;24:49-52. 91. Heydendael VMR, Spuls PI, Opmeer BC, n engl j med 352;18 www.nejm.org et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med 2003;349:658-65. 92. Granstein RD. New treatments for psoriasis. N Engl J Med 2001;345:284-7. 93. Mendonca CO, Burden AD. Current concepts in psoriasis and its treatment. Pharmacol Ther 2003;99:133-47. 94. Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest 2004;113:1664-75. 95. Boehncke WH. Immunomodulatory drugs for psoriasis. BMJ 2003;327:634-5. 96. van de Kerkhof PCM, de Hoop D, de Korte J, Cobelens SA, Kuipers MV. Patient compliance and disease management in the treatment of psoriasis in the Netherlands. Dermatology 2000;200:292-8. 97. Krueger GG, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol 2001;137: 280-4. 98. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999;41: 401-7. 99. Gottlieb AB, Lebwohl M, Shirin S, et al. Anti-CD4 monoclonal antibody treatment of moderate to severe psoriasis vulgaris: results of a pilot, multi-center, multiple-dose, placebo-controlled study. J Am Acad Dermatol 2000;43:595-604. 100. Asadullah K, Volk HD, Sterry W. Novel immunotherapies for psoriasis. Trends Immunol 2002;23:47-53. Copyright © 2005 Massachusetts Medical Society. may 5, 2005 The New England Journal of Medicine Downloaded from nejm.org on April 28, 2017. For personal use only. No other uses without permission. Copyright © 2005 Massachusetts Medical Society. All rights reserved.