* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenital Infections
Brucellosis wikipedia , lookup
Tuberculosis wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Chagas disease wikipedia , lookup
Hookworm infection wikipedia , lookup
Onchocerciasis wikipedia , lookup
Anaerobic infection wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
Toxoplasmosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Henipavirus wikipedia , lookup
Leptospirosis wikipedia , lookup
West Nile fever wikipedia , lookup
Sarcocystis wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Trichinosis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Herpes simplex wikipedia , lookup
Schistosomiasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Fasciolosis wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Hepatitis C wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
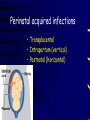
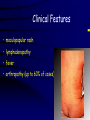
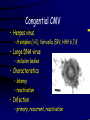
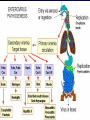
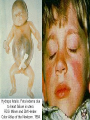
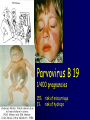
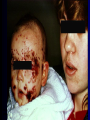
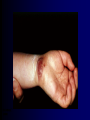
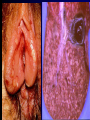
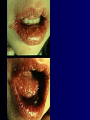
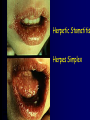
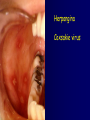
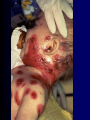
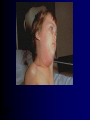
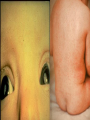
Infections in the Newborn and beyond Tony Ryan University College Cork Objectives 1. The common means of transmission of these infections. 2. The major manifestations of congenital and perinatal infections. 3. Diagnose and prevent these infections. Some pictures • The infectious diseases Lucky Box Infection in the Newborn • • • • Overall < 1% NICU 16% VLBW (<2500 g) 30% Mortality 30% Perinatal acquired infections • Transplacental • Intrapartum (vertical) • Postnatal (horizontal) Colonization • • • • Intrapartum (mothers flora) Postnatal (ward environment) CUMH 8500 deliveries Group B Streptococcus – 25% of mothers colonized (2000) – 50 % of babies colonized (1000) – 1-3% of babies infected (10-30) Preventing infection • Handwashing – before and after touching any baby – no watches, bracelets, nail varnish – sleeves rolled up – full scrub on arrival in NICU – 10 second wash in between – alcohol solutions ® © S.T.A.B.L.E. 2000 Congenital, Perinatal, and Neonatal Viral Infections TORCHS Intrauterine Viral Infections Rubella Cytomegalovirus (CMV) Parvovirus B19 Varicella-Zoster (VZV) Enteroviruses HIV HTLV-1 Hepatitis C Hepatitis B Lassa Fever Japanese Encephalitis Perinatal and Neonatal Infections Human Herpes Simplex VZV Enteroviruses HIV Hepatitis B Hepatitis C HTLV-1 Clinical Features • maculopapular rash • lymphadenopathy • fever • arthropathy (up to 60% of cases) Rubella History 1881 Rubella accepted as a distinct disease 1941 Associated with congenital disease (Gregg) 1961 Rubella virus first isolated 1967 Serological tests available 1969 Rubella vaccines available Risks of rubella infection during pregnancy Preconception minimal risk 0-12 weeks 100% risk of fetus being congenitally infected Spontaneous abortion occurs in 20% of cases. 13-16 weeks deafness and retinopathy 15% after 16 weeks normal development, slight risk of deafness and retinopathy Outcome Congenital Rubella • 1/3 rd will lead normal independent lives • 1/3 rd will live with parents • 1/3rd will be institutionalised Prevention Antenatal screening • Non-immune women vaccinated post partum • Effective live attenuated vaccine (95% efficacy) • Universal vaccination (MMR) • Selectively vaccination of schoolgirls Congential CMV • Herpes virus – H simplex (i/ii), Varicella, EBV, HHV 6,7,8 • Large DNA virus – inclusion bodies • Characteristics – latency – reactivation • Infection – primary, recurrent, reactivation Cytomegalovirus • member of the herpesvirus • primary infection usually asymptomatic. Virus then becomes latent and is reactivated from time to time. • transmitted by infected saliva, breast milk, sexually and through infected blood • 60% of the population eventually become infected. In some developing countries, the figure is up to 95%. Congenital Infection • Isolation of CMV from the saliva or urine within 3 weeks of birth. • Commonest congenital viral infection, affects 0.3 - 1% of all live births. • The second most common cause of mental disibility after Down's syndrome • Transmission to the fetus may occur following primary or recurrent CMV infection. 40% chance of transmission to the fetus following a primary infection. Cytomegalic Inclusion Disease • CNS abnormalities – - microcephaly, mental retardation, spasticity, epilepsy, – periventricular calcification. • Eye - choroidoretinitis and optic atrophy • Ear - sensorineural deafness • Liver - hepatosplenomegaly and jaundice which is due to hepatitis. • Lung - pneumonitis • Heart - myocarditis • Thrombocytopenic purpura, Haemolytic anaemia • Late sequelae in individuals asymptomatic at birth – hearing defects and reduced intelligence. Diagnosis • Isolation of CMV from the urine or saliva of the neonate. • Presence of CMV IgM from the blood of the neonate. • Detection of Cytomegalic Inclusion Bodies from affected tissue (rarely used) Management Of Congenital CMV • Primary Infection - termination of pregnancy? – 40% chance of the fetus being infected. – 10% chance that congenitally infected baby will be birth or develop sequelae later in life. symptomatic at – 4% chance (1 in 25) of giving birth to an infant with CMV problems. • Recurrent Infection - termination not recommended as risk of transmission to the fetus is much lower. • Antenatal Screening – impractical. • Vaccination - may become available in the near future. ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 Parvovirus B 19 1/400 pregnancies ® © S.T.A.B.L.E. 2000 15% 3% risk of miscarriage risk of hydrops Parvovirus • Causative agent of Fifth disease (erythema infectiosum), clinically difficult to distinguish from rubella. • Also causes aplastic crisis in individuals with haemolytic anaemias as erythrocyte progenitors are targeted. • Spread by the respiratory route, 60-70% of the population is eventually infected. • 50% of women of childbearing age are susceptible to infection. Congenital Parvovirus Infection • Known to cause fetal loss – hydrops fetalis; severe anaemia, congestive heart failure, generalized oedema and fetal death • No evidence of teratogenecity. • Risk of fetal death highest in second trimester (12%). • Minimal risk to the fetus in first or third trimesters • Maternal infection during pregnancy does not warrant termination of pregnancy. • Cases of diagnosed hydrops fetalis had treated in utero by intrauterine transfusions been successfully ® © S.T.A.B.L.E. 2000 Rotunda Hospital (1995-2002) 65 HIV positive women 2 infected babies with HIV protocol ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 Herpetic Stomatitis Herpes Simplex ® © S.T.A.B.L.E. 2000 Herpangina Coxsakie virus ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 Neonatal Varicella • VZV can cross the placenta in the late stages of pregnancy • Neonatal varicella may vary from a mild disease to a fatal disseminated infection. • If rash in mother occurs more than 1 week before delivery, then sufficient immunity would have been transferred to the fetus. • Zoster immunoglobulin should be given to susceptible pregnant women who had contact with suspected cases of varicella. • Zoster immunoglobulin should also be given to infants whose mothers develop varicella during the last 7 days of pregnancy or the first 14 days after delivery. ® © S.T.A.B.L.E. 2000 Mumps ® © S.T.A.B.L.E. 2000 Blueberry muffin baby ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 Prevention of Infection • • • • Reducing stress and overwork Controlling antibiotic use Encourage use of human milk Microbiological surveillance Any Questions? ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000 Adenoviruses cause Gastroenteritis Croup Lower respiratory tract infections ® © S.T.A.B.L.E. 2000 Summary CMV • 1% newborns infected (0.2% - 2.5%) • 90% asymptomatic • Higher infection rate with primary maternal (50%) than after recurrent maternal (1%) • Urine culture (gold standard) • worse outcome with – retinitis, microcephaly, neurological findings • Prevention is better than cure! Features of the Host • Portals of entry – (skin, cord, cannulae) • Host immunity – (poor local inflammatory response) • Antibiotic exposure • (superinfection, yeasts) • Prematurity – (immune-deficient, sicker) Congenital Viral Infections An Overview Congenital Rubella Syndrome Classical triad consists of cataracts, heart defects, and sensorineural deafness. Many other abnormalities had been described and these are divided into transient, permanent and developmental. Transient low birth weight, hepatosplenomegaly, thrombocytopenic purpura bone lesions, meningoencephalitis, hepatitis, haemolytic anemia pneumonitis, lymphadenopathy Permanent Sensorineural deafness, Heart Defects (peripheral pulmonary stenosis, pulmonary valvular stenosis, patent ductus arteriosus, ventricular septal defect) Eye Defects (retinopathy, cataract, microopthalmia, glaucoma, severe myopia) Other Defects (microcephaly, diabetes mellitis, thyroid disorders, dermatoglyptic abnormalities Developmental Sensorineural deafness, Mental retardation, Diabetes Mellitus, thyroid disorder Disease is spread by • Faecal - oral route – gastroenteritis • Respiratory (coughing) – colds, viruses • Person to person – Streptococcus, cold sores, scabies • Contact with blood, urine, saliva – CMV, hepatitis Incidence of Cytomegalic Disease U.S.A. U.K. No. of live births p.a. 3,000,000 700,000 Rate of congenital CMV 1% 0.3% No. of infected infants 30,000 2100 Symptomatic at birth (5 - 10% ) 1,500-3,000 105 Fatal disease (~ 20% ) 300-600 22 No. with sequelae (90% of survivors) 1080-2160 83 Asymptomatic (90 - 95% ) 27000 1995 No. with late sequelae 1350-4550 315 Characteristics of Rubella • RNA enveloped virus, member of the togavirus family • Spread by respiratory droplets. • In the prevaccination era, 80% of women were already infected by childbearing age. Infection increased by certain features of microorganisms • Pathogenicity – GBS, CONS, E.Coli, etc. • Dose – heavy colonization increases infection • Competition – e.g. inhibition of yeasts by bacteria Laboratory Diagnosis Diagnosis of acute infection • Rising titres of antibody (mainly IgG) • Presence of rubella-specific IgM - Typical Serological Events following acute rubella infection level Neonatal Herpes Simplex (1) • Incidence of neonatal HSV infection varies inexplicably from country to country e.g. from 1 in 4000 live births in the U.S. to 1 in 10000 live births in the UK. • The baby is usually infected perinatally during passage through the birth canal. • Premature rupturing of the membranes is a well recognized risk factor. • The risk of perinatal transmission is greatest when there is a florid primary infection in the mother. • There is an appreciably smaller risk from recurrent lesions in the mother, probably because of the lower viral load and the presence of specific antibody. • The baby may also be infected from other sources such as oral Neonatal Herpes Simplex (2) • The spectrum of neonatal HSV infection varies from a mild disease localized to the skin to a fatal disseminated infection. • Infection is particularly dangerous in premature infants. • Where dissemination occurs, the organs most commonly involved are the liver, adrenals and the brain. • Where the brain is involved, the prognosis is particularly severe. The encephalitis is global and of such severity that the brain may be liquefied. • A large proportion of survivors of neonatal HSV infection have residual disabilities. • Acyclovir should be promptly given in all suspected cases of neonatal HSV infection. • The only means of prevention is to offer caesarean section to mothers with florid genital HSV lesions. Varicella-Zoster Virus • 90% of pregnant women already immune, therefore primary infection is rare during pregnancy • Primary infection during pregnancy carries a greater risk of severe disease, in particular pneumonia First 20 weeks of Pregnancy up to 3% chance of transmission to the fetus, recognised congenital varicella syndrome; • Scarring of skin • Hypoplasia of limbs • CNS and eye defects • Death in infancy normal Prevention of Infection • • • • Reduce contact Breast feeding Early discharge home Environment – Unit design – Equipment (own stethescope, thermometer, humidification, ventilators) • Controlling admissions (transitional care) Prevention of Infection • Cleanliness of baby • Cord care – dry care vs alcohol swabs • Other babies, staff, visitors – RSV, varicella zoster • Invasive procedures – sterile technique Cytomegalovirus CMV Epidemiology of CMV • Shed – body secretions, urine • Transfer – – – – – – Intimate contact transplacental during birth breast feeding blood products organ transplantation CMV in the newborn • 1% newborns infected (0.2% - 2.5%) • Higher infection rate with primary maternal (50%) than after recurrent maternal (1%) • 50% of babies fed CMV infected breast milk get infection CMV in Toddlers • • • • Day care Can shed for up to a year Plastic toys Hands of day care workers CMV in Pregnancy • 90% of women asymptomatic • Primary infection 1 - 4% of pregnancies • Foetal transmission 25 - 75% (~ 40%) • More neuro sequelae in infants of primary infection • Maternal antibody does not protect against infection but may be associated with less sequelae Diagnosis in Pregnancy • • • • Usually asymptomatic Sometimes ‘flu-like illness (like EBV) Conversion to IgG positive Rising titres not helpful CMV and foetus • Transmission can occur in all trimesters • Adverse neuro outcome more likely in 1st trimester infection Oligohydramnios Microcephaly polyhydramnios dilated ventricles IUGR calcification non-immune hydrops pseudomeconium ileus ascites effusions Diagnosis in foetus • Remember most foetuses look normal • Ultrasound findings • CMV IgM (sensitivity = 75%) – negative result does not exclude infection • Amniocentesis/cordocentesis – culture/PCR – LFTs, thrombocytopenia, leucopenia • Antenatal counseling difficult CMV and newborn • 90% newborn asymptomatic • 10% have clinical signs SGA hepatosplenomegaly microcephaly jaundice calcification Petechiae chorioretinitis thrombocytopenia hearing loss meningitis Lab diagnosis in newborn • Urine culture (gold standard) – positive in first 3 weeks suggests congenital • CMV IgM (sensitivity = 75%) – negative result does not exclude infection • CMV PCR (urine) • LFT’s, thrombocytopenia, anaemia, leucopenia Long term Follow-up essential “When the rockets go up Who cares where they come down That’s not my department Says Werner von Braun” CMV in older children/adolescents • • • • • Intrafamilial sexual contact blood products Day care workers Socioeconomic – developing countries (80% of 3 year olds; 100% of adults) – UK/USA upper SE class 40-60%; lower SE class 80% Prevention of CMV • • • • Vaccine Focus on high-risk (childbearing) Education CMV negative blood Follow up • • • • Neuro-developmental Opthalmology Hearing (BAER) Educational – dyspraxia – learning difficulties Better outcome • Reticulo-endothelial involvement • When CNS not involved Worse outcome •Chorio-Retinitis •Microcephaly •Early neurological findings ‘Silent’ CMV outcome • Hearing loss 15% • mostly normal neurologically Infectious diseases (U.S.) pre vaccination and 1997 Diptheria (1921) 206,939 5 Measles (1941) 894,134 135 Mumps (1968) 152,209 135 Pertussis (1934) 265,269 5,519 Polio (1952) 21,269 0 Rubella (1968) 57,686 161 Tetanus (1948) 1560 43 TB (1948) 20,000 165 ® © S.T.A.B.L.E. 2000 ® © S.T.A.B.L.E. 2000