* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Disinfectant
Psychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Antibiotics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
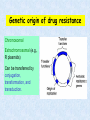
Sterilization, Disinfection and Antibacterial Agents • Pin Lin (凌 斌), Ph.D. Departg ment of Microbiology & Immunology, NCKU ext 5632 [email protected] • References: 1. Chapters 8 & 20 in Medical Microbiology (Murray, P. R. et al; 5th edition) 2. 醫用微生物學 (王聖予 等編譯, 4th edition) Outline 1. Sterilization (Definition & Methods) 2. Disinfection (Definition & Methods) 3. Mechanisms of Antimicrobial Action 4. Antibacterial Agents What is Sterilization? Sterilization (in Microbiology) : 1. To completely remove all kinds of microbes (bacteria, mycobacteria, viruses, & fungi) by physical or chemical methods. 2. Effective to kill “bacterium spores” 3. Sterilant: material or method used to remove or kill all microbes Methods of sterilization (I) Not for virus Methods of sterilization (II) Sterilize instruments Physical methods (moist heat, dry heat, filtration, radiation) Moist heat Boiling: boiling for 10 min => Kill most vegetative forms of bacteria Longer time => Kill spores Addition of 2% Na2CO3 or 0.1% NaOH => enhance destruction of spores and prevent rusting of the metal wares. Low temperature disinfection (Pasteurization): 62-65 oC for 30 min. or 71.5 oC for 15 sec. This is mainly used for disinfection of milk. Autoclave: 121-132 oC for 15 min or longer => Kill both the vegetative and spore forms of the bacteria. => Use Bacillius stearothermophilus spores to monitor the effectiveness of Autoclave Dry heat Dry oven: 160 oC for 2 hrs or 171 oC for 1 hr. (B. subtilis) Flaming; incineration Radiation UV-light: UV-radiation causes damage to DNA. Ionizing radiation: less applicable. Filtration The pore size for filtering bacteria, yeasts, and fungi is in the range of 0.22-0.45 mm (filtration membranes are most popular for this purpose). HEPA filters Chemical methods Alcohol: protein denaturant. 70% aqueous solution of ethyl alcohol and isopropyl alcohol are commonly used as skin disinfectants. Phenolics: Phenol and phenolic compounds (e.g. lysol) lyse the cell membrane and denature proteins at 1-2% (aqueous solution). Oxidizing agents: inactivate cells by oxidizing free sulfhydryl group, e.g., peracetic acid, iodine, iodophore, H2O2 (3-6%), hypochlorite, and chlorine etc. Plasma gas sterilization: H2O2 vapors treated with microwave or radio energy to produce reactive free radicals; no toxic byproducts. An efficient sterilization for dry surfaces. Alkylating agents Formalin (37% aqueous solution of formaldehyde), glutaraldehyde Ethylene oxide gas (made nonexplosive by mixing with CO2 or a fluorocarbon): a reliable disinfectant for dry surfaces. Detergents: surface-active agents that disrupt the cell membranes. Anionic detergents: e.g. soaps, and bile salts. Cationic detergents: e.g., the quaternary ammonium compounds, are highly bactericidal for both the gram-positive and negative bacteria in the absence of contaminating organic matter. Pros & Cons of Sterilants (I) 1. Steam (Moist) & Dry Heat => the most common methods for most materials. Cons: No good for heat-senstive, toxic or volatile chemicals 2. Filtration => remove bacteria and fungi from air or solutions eg.: HEPA (High-Efficiency Particular Air) filters Cons: unable to remove viruses and some small bacteria (microplasma) 3. Ethylene oxide => the most common gas vapor sterilant Cons: (1) flammable & explosive (2) potential carcinogenic 4. Formaldehyde gas => carcinogenic Pros & Cons of Sterilants (II) 5. Plasma gas => Hydrogen peroxide => Reactive free radicals Microwave - or => No Toxic byproducts radio-freq energy => may replace many applications for ethylene oxide Cons: NOT good for materials absorbing or reacting with H2O2 6. Peracetic acid => an oxidizing agent w/ good activity => end products nontoxic 7. Glutaraldehyde => Not safe What is Disinfection? Disinfection (in Microbiology) : 1. To kill most of microbial forms except some resistant organisms or bacterium spores 2. Categorizing: High-level sterilization Intermediate-level Low level Not effective for all bacteria or spores 3. Disinfectant: a substance or method used to kill microbes on surfaces High-level disinfectants Used for items involved in invasive procedures but NOT withstand sterilization, e.g. Endoscopes, Surgical instruments Intermediate-level disinfectants Used for cleaning surface or instruments without bacterial spores and highly resilient organism, eg. Laryngoscopes, Anesthesia breathing circuits…etc Low-level disinfectants Used to treat non-critical instruments and devices, not penetrating into mucosa surfaces or sterile tissues Considerations of Disinfection Effectiveness of disinfectants is influenced by: 1. Nature of the item to be disinfected 2. Number and resilience of the contaminants 3. Amount of organic material present 4. Type and concentration of disinfectant 5. Duration and temperature of exposure Antisepsis & Antiseptic agents 1. Use of chemical agents on skin or living tissues to inhibit or eliminate microbes 2. Antiseptic agents are selected for their safety & efficacy Outline • Introduction • Sterilization (Definition & Methods) • Disinfection (Definition & Methods) • Mechanisms of Antimicrobial Action The Discovery of Antibacterial Agents 1. In 1930s Gerhard Domagk discovered the antibacterial effect of prontosil (=> sulfanilamide) => 1939 Nobel Laureate 2. A. Fleming discovered that the mold Penicillium prevented the multiplication of staphyloocci. => The first antibiotic, Penicillin, was identified => 1945 Nobel Laureate 3. Streptomycin, tetracyclines & others were thereafter developed to treat infectious diseases. 4. Bacteria start developing resistance to these agents. Mechanisms of Antibacterial agents 1. A useful chemotherapeutic agent should have in vivo effectiveness and selective toxicity. 2. Modes of action of the chemotherapeutic agents Inhibition of: - Cell wall synthesis - Protein synthesis - Nucleic acid synthesis - Cell membrane function Sites of Action of Antibacterial Chemical Agents Peptidoglycan 1. A major component of cell wall 2. Forms a meshlike layer consisting: a polysaccharide polymer cross-linked by Peptide bonds 3. Cross-linking reaction is mediated by: Transpeptidases DD-carboxypeptidases => Targets of Penicillin Inhibition of cell wall synthesis (Penicillins, Cephalosporins, Vancomycin, Cycloserine, Bacitracin) b-lactam drugs: Drugs containing a b-lactam ring, e.g. penicillins and cephalosporins. Vancomycin: bactericidal for some gram-positive bacteria PBPs (penicillin-binding proteins): receptors for b-lactam drugs. There are 3-6 PBPs, some of which are transpeptidation enzymes. Outline • Introduction • Sterilization (Definition & Methods) • Disinfection (Definition & Methods) • Mechanisms of Antimicrobial Action • Antibacterial Agents Penicillins Produced by Penicillium chrysogenum Modifications: decrease acid lability; increase absorption; resistant to penicillinase; broader spectrum (e.g., Ampicillin). b-lactamase inhibitors: bind b-lactamases irreversibly; combined use with some penicillins to increase effectiveness. Modifications of cephalosporins were to expand their spectra or increase their stability to b-lactamases. Vancomycin 1. A complex glycopeptide produced by Streptomyces orientalis 2. Interacts with D-ala-D-ala termini of the pentapeptide side chains 3. Inactive for gram-negative bacteria 4. Some enterococci have acquired resistance to vancomycin 5. The resistance genes are carried on plasmids. Polymyxins 1. Cyclic polypeptides (from Bacillus polymyxa) 2. Insert into bacteria outer membrane by interacting with LPS and phospholipids Increase cell permeability bacterial cell death 3. Most Active for gram-negative bacteria, because Grampos bacteria have no outer member 4. Nephrotoxic 5. External treatment of localized infection and oral administration to sterilize the gut. Drug resistance of the microbes 1. Producing enzymes that degrade or modify the active drugs 2. Decreasing drug entry 3. Increasing drug efflux 4. Increasing the amount of target enzyme 5. Decreasing affinity of target for drug 6. Developing an altered metabolic pathway that bypass the reaction inhibited by the drug How Bacteria Become Resistant to the b-Lactam Antibiotics? 1. To prevent the interaction between the antibiotic and the target PBP e.g. Gram-neg (Pseudomonas) => change porins on the pores => exclude antibiotic 2. To modify the binding of the antibiotic to the target PBP Modified PBP can result from mutation or acquisition of new PBP 3. Hydrolysis of the antibiotic by b-lactamases - They are in the same family of PBPs - Over 200 different b-lactamases: some are specific for penicillins others have a broad range of activity Outer wall of Gram-positive and Gram-negative species Inhibition of protein synthesis Aminoglycosides (streptomycin, kanamycin, neomycin, gentamicin, tobramycin, amikacin, etc.): bind irreversibly to 30S ribosomal proteins and inhibit peptide formation; bactericidal. Tetracyclines: inhibit attachment of charged tRNA; bacteriostatic. Chloramphenicol: binds to peptidyl transferase of ribosome; toxic to bone marrow cells (aplastic anemia); bacteriostatic. Macrolides (erythromycins, clarithromycin, etc.): bind to 23S rRNA and block peptide elongation. Lincomycins (clindamycin): resembles macrolides in mode of action. Oxazolidinones (linezolid): blocks formation of imitiation complex. Active against staphylococci, streptococci and enterococci. No crossresistance with the above antibiotics. Reserved for multidrug-resistant enterococci. Resistance to aminoglycosides: 1. Mutation to ribosomal binding site; 2. Decreased uptake of antibiotic; 3. Enzymatic modification of the antibiotic. Inhibition of nucleic acid synthesis quinolones, rifampin, sulfonamides, trimethoprime, pyrimethamine Rifampin: inhibits RNA synthesis. Quinolones and fluoquinolones: blocking DNA gyrase. Metronidazol: effective to anaerobic bacterial infections. Reduction of its nitro group by bacterial nitroreductase produces cytotoxic compound that disrupts bacterial DNA. Antimetabolites Sulfonamides: analogs of p-aminobenzoic acid (PABA) and inhibit synthesis of folic acid, which is an important precursor to the synthesis of nucleic acids. Trimethoprim: inhibits dihydrofolic acid reductase in synthesis of purines, methionine and glycine. Antimicrobial activity in vivo Factors affecting the effectiveness of antibiotics in vivo Environment Amount of pathogen State of bacterial metabolic activity Distribution of drug Location of organisms Interfering substances Concentration of antibiotic Absorption Distribution Variability of concentration Dangers of indiscriminate use of antibiotics 1. Development of drug resistance. 2. “Superinfection" resulting from changes in the normal flora of the body. 3. Masking serious infection without eradicating it. 4. Drug toxicity. 5. Widespread sensitization of the population with resulting hypersensitivity, anaphylaxis, rashes, fever, blood disorders, cholestatic hepatitis, and perhaps collagen-vascular diseases. Genetic origin of drug resistance Chromosomal Extrachromosomal (e.g., R plasmids) Can be transferred by conjugation, transformation, and transduction. General rules in antimicrobial therapy 1. Give an antimicrobial drug only when it is indicated by rational choices 2. Give a sufficiently large amount of an effective drug as early as possible 3. Continue treatment long enough to ensure eradication of infection Limitation of drug resistance 1. Maintain sufficiently high levels of the drug in the tissue to inhibit both the original population and first-step mutants. 2. Simultaneously administer two drugs that do not give cross-resistance. 3. Avoid exposure of microbes to a particular drug by limiting its use, especially in hospitals and in animal feeds. Cross-resistance: microbes resistant to a certain drug may also be resistant to other drugs that share a mechanism of action. (e.g., different aminoglycosides, macrolides, and lincomycins) Selection of antibiotics Diagnosis Antibiotic susceptibility tests Antimicrobial drugs used in combination Indications Prompt treatment of patients suspected of having a serious microbial infection. To delay the emergence of mutants resistant to one drug in chronic infections. To treat mixed infections. To achieve bactericidal synergism or to provide bactericidal action. Disadvantages Relaxation of the effort to establish a diagnosis. Greater chance for adverse reactions. Unnecessary cost. Not necessarily effective than single drug treatment. Antagonism between drugs (rarely). Effects of combined usage of two antibiotics Indifference (A + B=A or B) Addition (A + B=A + B) Synergism (A + B=A x B) Antagonism (A + B= 0 or less) SUMMARY-I 1. Various antimicrobial agents act by interfering with: (1) cell wall synthesis, (2) plasma membrane integrity, (3) nucleic acid synthesis, (4) ribosomal function, and (5) metabolite synthesis. 2. Cell wall synthesis is inhibited by ß-lactams, such as penicillins and cephalosporins, which inhibit peptidoglycan polymerization, and by vancomycin, which combines with cell wall substrates. SUMMARY-II 3. Bacteria can evolve resistance to antibiotics. Resistance factors can be encoded on plasmids or on the chromosome. Resistance may (1) decreased entry of the drug, (2) changes in the receptor (target) of the drug, or (3) metabolic inactivation of the drug. 4. Combinations of antibiotics may act synergisticallyproducing an effect stronger than the sum of the effects of the two drugs alone or antagonistically, if one agent inhibits the effect of the other. Disinfection and Sterilization Disinfection: killing of most microbial forms. Disinfectant: a chemical substance used to kill microbes on surfaces but too toxic to be applied directly to tissue. Antisepsis: inhibit or eliminate microbes on skin or other living tissue Sterilization: removal of life of every kind by physical or chemical methods. Sterilant: an agent or method used to remove or kill all microbes. Septic: presence of pathogenic microbes in living tissue. Aseptic: absence of pathogenic microbes. Sterile: free of life of every kind. Bacteriostatic: inhibiting bacterial multiplication. Bacteriostatic action is reversible by removal or inactivation of agent. Bactericidal: killing bacteria. Modes of action of antimicrobial agents 1. Damage to DNA Formation of pyrimidine dimer by UV irradiation Single- or double-strand DNA break by ionizing radiation DNA reactive chemicals, e.g. alkylating agents 2. Protein denaturation 3. Disruption of cell membrane or wall 4. Removal of free sulfhydryl groups Formation of disulfide bond by oxidizing agents Heavy metals combine with sulfhydryls 5. Chemical antagonism: interference with the normal reaction between an enzyme and its substrate. Sites of Action of Antibacterial Chemical Agents