* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry – Summary of Reactions and Conditions
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Asymmetric induction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Aldol reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Petasis reaction wikipedia , lookup
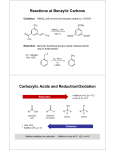
565323042 Organic Chemistry Reactions and Conditions Alkane 1 Combustion in the presence of oxygen General equation for non-cyclic alkanes CnH(2n+2) + (1½n+½)O2 nCO2 + (n+1)H2O Conditions: Ignition in the presence of plentiful oxygen. Gives a cleaner flame than unsaturated hydrocarbons. Flame becomes increasingly sooty with increasing chain length. In limiting oxygen, products may include C and CO. 2 Conversion to halogenoalkane Reaction type: Free radical substitution C2H6 + Cl2 C2H5Cl + HCl Reagents: Conditions: chlorine or bromine Ultraviolet light Gas or liquid phase (inert solvent such as CCl4) Further substitutions usually occur. Knowledge of mechanism required. Alkene 1. Conversion to halogenoalkane Reaction type: Electrophilic addition H H H H C C H Reagents: Conditions: + HCl H C C H H H Cl hydrogen halide Room temperature Gas or liquid phase, or inert solvent (tetrachloromethane) Markovnikov’s rule: When hydrogen halide adds to an unsymmetrical double bond, the hydrogen adds to the carbon atom containing most hydrogen atoms. Knowledge of mechanism and explanation of Markovnikov’s rule is required. 2. Conversion to dihalogenoalkane Reaction type: Electrophilic addition 1/16 565323042 H H H H C C H + Cl2 H C C H H Reagents: Conditions: Cl Cl chlorine or bromine room temperature, in dark. gas phase or inert solvent (tetrachloromethane). Knowledge of mechanism required. 3. Conversion to alkyl sulphate and then to an alcohol (i) Addition of sulphuric acid Reaction type: Electrophilic addition H H H H + H2 SO4 C C H H C C O S OH H H H Reagents: Conditions: O O concentrated sulphuric acid. 0°C Addition to unsymmetrical double bond follows Markovnikov’s rule. (ii) Conversion of alkyl sulphate to an alcohol Reaction type: hydrolysis H H O H C C O H H S OH + H2O HO C C H O Reagents: Conditions: 4. H H + H2SO4 H H water warm under reflux Conversion to diol Reaction type: oxidation H H H C C + 2 KOH + 2 KMnO4 HO C C OH + 2 K2MnO4 H H H H H Reagents:: Conditions: 5. dilute aqueous alkaline potassium manganate (VII) room temperature Conversion to alkane Reaction type: addition H + H2 C C H H H H H Reagents: Conditions: H C C H H H hydrogen nickel catalyst, 100C, 4 atm 2/16 565323042 6. Polymerisation H C C n H H H H H C C n H H Reagents and conditions: Either Free radical initiator such as organic peroxide, 100-300C, 1000-2000 atm (low density polyethene produced, free radical mechanism) or Ziegler catalyst, 20-50C, 5-25 atm (high density polyethene produced, ionic mechanism) Halogenoalkane 1. Conversion into alcohol Reaction type: nucleophilic substitution H H H H H C C Cl + NaOH H C C OH H H Reagents: Conditions: + NaCl H H Aqueous sodium or potassium hydroxide. Boil under reflux. Knowledge of possible mechanisms required. Note the difference in reagent and conditions in reaction 3. H2O has a lone pair of electrons and can act as a nucleophile in a similar way to OH -. Lacking an overall negative charge, H2O is a weaker nucleophile than OH-. One method of following the reaction of a halogenoalkane with water is to carry out the reaction in the presence of AgNO3 (aq). The Ag+ (aq) ions do not take part in the organic reaction. They do combine with the halide ion product and the formation of a silver halide precipitate provides an indication of the progress of the reaction. 2. CH3CH2Cl + H2O CH3CH2OH + Cl- (aq) + Ag+ (aq) H+ (aq) + Cl- (aq) AgCl (s) Conversion into amine Reaction type: nucleophilic substitution H H H H H C C Br + 2 NH3 H C C NH2 + NH4Br H H Reagents: Conditons: H H Excess ammonia dissolved in ethanol. Heat in a sealed tube. Amines, having a lone pair of electrons on the nitrogen atom like ammonia, are themselves nucleophiles. Therefore secondary and tertiary amines and quartenary amine salts also tend to be formed which limits the usefulness of this reaction in synthesis. CH3CH2Br + CH3CH2NH2 CH3CH2Br + (CH3CH2)2NH (CH3CH2)2NH + HBr (CH3CH2)3N + HBr 3/16 565323042 CH3CH2Br + (CH3CH2)3N 3. (CH3CH2)4N+ Br - Conversion into alkene Reaction type: Elimination (Acid/base) H H H H C C Cl + KOH H C C H H H + KCl + H2O H Reagents: Potassium hydroxide dissolved in ethanol Conditions: Boil under reflux. If several products are possible, the double bond tends to form between carbon atoms which have fewest hydrogen atoms attached. Note the difference in reagent and conditions in reaction 1. 4. Alkylation (Friedel-Crafts reaction) Reaction type: Electrophilic substitution + RCl Reagents: Conditions: R + HCl benzene and anhydrous AlCl3 (catalyst). heat under reflux. Attachment of an alkyl group activates the arene ring, making it susceptible to further alkylations. These occur at positions 2 and 4. 5. Preparation of a nitrile CH3CH2Br + KCN CH3CH2C≡N + KBr Reaction type : Mechanism: Conditions: Substitution Nucleophilic (CN- is nucleophile) Heat under reflux in ethanol + water solvent Alcohol 1 Formation of alcoxide salt Reaction type: Redox 2 CH3CH2OH + 2 Na 2 CH3CH2O- Na+ + H2 Reagents: Alkali metal Conditions: Room temperature. The alcoxide salt can be used to prepare an ether by reaction with a halogenoalkane. 2. 4/16 565323042 (a) Conversion of primary alcohol to aldehyde Reaction type: Oxidation H H H O 3 H C C OH + Cr2O72- + 8 H+ 3 H C C H + 2 Cr3+ + 7 H2O H H Reagents: Conditions: (b) H aqueous potassium dichromate (VI), acidified with dilute sulphuric acid. alcohol reactant added to hot oxidation reactant, aldehyde product instantly distilled to prevent further oxidation to carboxylic acid. Conversion of primary alcohol to carboxylic acid Reaction type: Oxidation H H H O 3 H C C OH + 2 Cr2O72- + 16 H+ 3 H C C OH + 4 Cr3+ + 11 H2O H H Reagents: Conditions: (c) H aqueous potassium dichromate (VI), acidified with dilute sulphuric acid heat under reflux, then distill Conversion of secondary alcohol to ketone Reaction type: Oxidation OH O 3 H3C C CH3 + Cr2O72- + 8 H+ 3 H3C C CH3+ 2 Cr3+ + 7 H2O H Reagents: Conditions: (d) 3. potassium dichromate (VI), acidified with dilute sulphuric acid. heat under reflux. The ketone produced is resistant to further oxidation. Tertiary alcohols are resistant to oxidation. Formation of ester Reaction type: Condensation (nucleophilic addition followed by elimination) H H H O H C C OH + H C C OH H H Reagents: Conditions: 4. H O H H C C O C C H + H2O H H H Appropriate pure carboxylic acid, small amount of conc. sulphuric acid Heat and distill Conversion to alkene Reaction type: Elimination H H H C C OH H H 5 H H H H C C H + H2O H Reagents: excess concentrated sulphuric acid Conditions: heat to 170C Where several products are possible, those in which the carbon atoms of the double bond contain most alkyl groups are favoured. Conversion to halogenoalkane Reaction type: Substitution 5/16 565323042 Several reagents may be used. Conversion to chloroalkane CH3CH2OH + PCl5 CH3CH2Cl + POCl3 + HCl Reagents: phosphorus (V) chloride Conditions: room temperature CH3CH2OH + SOCl2 CH3CH2Cl + SO2 + HCl Reagents: thionyl chloride (sulphur oxide dichloride) Conditions: room temperature Conversion to bromoalkane CH3CH2OH + HBr CH3CH2Br + H2O Reagents: 50% sulphuric acid and sodium bromide. Conditions: Heat under reflux. The hydrogen bromide is generated by the reaction between sodium bromide and sulphuric acid NaBr + H2SO4 HBr + NaHSO4. Conversion to bromo- or iodoalkane 3CH3CH2OH + PBr3 3CH3CH2Br + H3PO3 3CH3CH2OH + PI3 3CH3CH2I + H3PO3 Reagents: Conditions: moist red phosphorus and bromine or iodine warm phosphorus (III) halide is formed as the halogen reacts with the phosphorus. Carbonyl Compounds (Aldehydes and Ketones) 1. Conversion of aldehyde to carboxylic acid Reaction type: oxidation Preparation of carboxylic acid H O H O 3 H C C H + Cr2O72- + 8 H+ 3 H C C OH H H Reagents: Conditions: + 2 Cr3+ + 7 H2O aqueous potassium dichromate (VI) and dilute sulphuric acid heat under reflux Benedict's test for an aldehyde (not a preparative method) H O H C C H H O + 2 Cu2+ + 5 OH- H H C C O- + Cu2O + 3 H2O H Warm with Benedict's reagent. Formation of a red / brown precipitate indicates the presence of an aldehyde. Fehling's solution behaves in a similary way. Both Benedict's and Fehling's 6/16 565323042 contain copper (II) complexes. These complexes enable Cu (II) to remain in solution in the presence of alkali. Ketones are resistant to oxidation and do not react with Benedict's solution. Tollen's reagent (ammoniacal silver nitrate) (not a preparative method) Prepared by adding excess ammonia solution to silver nitrate solution so that the initially formed precipitate redissolves. The solution formed contains Ag(NH 3)2+ (aq). Aldehydes are oxidised by this reagent but not ketones: RCHO + 2 Ag(NH3)2+ (aq) + 3 OH- (aq) RCOO- + 2 Ag (s) + 4 NH3 (aq) + 2 H2O (l) Formation of a silver mirror on the test tube indicates the presence of the aldehyde group. 2. Formation of 2,4 dinitrophenylhydrazone Reaction type: nucleophilic addition followed by elimination O2N H O NH NH2 O2N H H + H C C H NH N C C H H NO2 NO2 O2N + H2O H O2N O NH NH2 + H3C C CH3 CH3 NH N C NO2 Reagents: Conditions: NO2 + H2O CH3 2,4 dinitrophenylhydrazine in concentrated hydrochloric acid or concentrated phosphoric acid (H3PO4) Room temperature 2,4 dinitrophenylhydrazones are characteristically orange crystalline solids at room temperature. Determination of their melting points is often used to identify the aldehyde or ketone from which they were prepared. 3 Reduction with LiAlH4 (lithium tetrahydridoaluminate) LiAlH4 reduces an aldehyde to a primary alcohol and a ketone to a secondary alcohol. The reaction conditions are the same as those used in the reduction of a carboxylic acid by LiAlH 4. 4 Addition of hydrogen cyanide to aldehydes and ketones Reaction type: Mechanism: addition nucleophilic OH O + CH3 C HCN CH3 CH3 C CH3 CN The highly toxic HCN gas is generated by adding dilute sulphuric acid to KCN. The actual reaction mixture should be pH 8. The cyanohydrin product can be converted into a -hydroxy carboxylic acid by heating with concentrated HCl (aq) under reflux. 7/16 565323042 OH CH3 OH CH3 + C 2 H2O + H+ CH3 C 2 NH4+ COO- CN 5 CH3 + Reaction with iodine in the presence of alkali Conditions: KI (aq) in the presence of NaOH (aq) In these conditions, I- disproportionates to form IO-. R O H C C H + 3 IO- R O I C C H R O I C C I + 3 OH- I RCO2- + CHI 3 I + OH- I The CHI3 product of this reaction is a characteristic pale yellow precipitate. This reaction is used to test for the -COCH3 and -CHOHCH3 groupings (secondary alcohol is initially oxidised to the ketone). Carboxylic Acid 1. Formation of ester Reaction type: Condensation (nucleophilic addition of alcohol to acid followed by elimination of water) H H H O H C C OH + H H Reagents: Conditions: 2. H H H C C OH H O H H C C O C C H + H2O H H H alcohol and concentrated sulphuric acid. heated under reflux to form equilibrium mixture. Conversion to alcohol Reaction type: Reduction H O 4 H C C OH + 3 LiAlH4 LiAl (OCH2CH3)4 + 4 H2 + 2 LiAlO2 H LiAl(OCH2CH3)4 + 4 H2O 4 CH3CH2OH + Al(OH)3 + LiOH These equations do not need to be learnt. The reducing agent can be described notionally in the equation as [H]. H O H C C OH H H H + 4 [H] H C C OH H H 8/16 + H2O 565323042 Reagents and conditions: i) Lithium tetrahydridoaluminate (III) (lithium aluminium hydride) in dry ether solvent ii) Water (caution – hydrogen released / fume cupboard) It is easier to reduce an ester than a carboxylic acid. Therefore, a carboxylic acid may be first esterified with an alcohol and then reduced as above (to form 2 alcohols). H H O H H H H C C O C C H H H + 4 [H] 2 H C C OH H H H It is not possible to produce an aldehyde directly from a carboxylic acid by reduction. 3. Conversion to acid chloride Reaction type: Substitution H O H O + PCl5 H C C Cl H C C OH H H Reagents: Conditions: 4. + POCl3 + HCl phosphorus (V) chloride room temperature Conversion to a salt Reaction type: Acid-base H O H O H C C OH + NaOH H C C O- Na+ H 2 H + H2O H H OH C C O + Na2CO3 (aq) 2H H H O-Na+ C C O + CO2 (g) + H2O (l) H Reagents: Conditions: aqueous alkali room temperature Carboxylic Esters 1. Conversion to alcohol and carboxylic acid Reaction type: Hydrolysis (addition of water followed by elimination of alcohol) i) Acid catalysed H H O H H C C O C C H H H Reagents: Conditions: H H + H2O H C C OH + H H H dilute sulphuric acid heat under reflux 9/16 H O H C C OH H 565323042 The reaction reaches equilibrium – simply the reverse of ester synthesis from carboxylic acid and alcohol. ii) In alkali H H O H H H H C C O C C H H H + OH- H C C OH + H C C O- H Reagents: Conditions: H O H H H aqueous sodium or potassium hydroxide heat under reflux Unlike acid catalysed hydrolysis, the reaction goes to completion. The carboxylate salt is converted into the protonated acid by adding dilute hydrochloric acid. This is how soaps are made from triglycerides. O O H H C O C R' O + O O + - O O + - Na H C O H H C O C R'' + 3 NaOH H C O H H C O O H C R''' H C O H - C R'' Na C R''' H Na triglyceride 2 C R' H glycerol O fatty acid salts Transesterification (i) Reaction with a carboxylic acid Reaction type: substitution Mechanism: nucleophilic Acid catalyst required CH3COOCH2CH3 + HCOOH ⇌ HCOOCH2CH3 + CH3COOH This type of reaction is used to increase the proportion of saturated acids (e.g. stearic acid) in margarine to increase its melting point. (ii) Reaction with an alcohol CH3COOCH2CH3 + CH3OH CH3COOCH3 + CH2CH3OH A catalyst such as solid sodium hydroxide is required for this reaction. Biodiesel is made from vegetable oils but the triglycerides in the oils need to be converted into methyl or ethyl esters in order for the oil to be a suitable fuel for the engine. e.g. O CH2O HCO C C R O R CH2OH O + 3 CH3OH 3 + CH3O CH2O C C CHOH R CH2OH R O 10/16 565323042 Acyl Chloride (Acid chloride) 1. Conversion to carboxylic acid Reaction type: hydrolysis H O H O H C C Cl + H2O H C C OH H H Reagents: Conditions: 2. water room temperature, reaction is vigorous Conversion to ester Reaction type: addition followed by elimination H H H O H C C Cl + H C C OH H H Reagents: Conditions: H H Conversion to amide Reaction type: addition followed by elimination H O H C C Cl H Reagents: Conditions: + 2 NH3 H C C NH2 + NH4Cl H aqueous ammonia. room temperature (care needed). Conversion to N-substituted amide Reaction type: addition followed by elimination H O H O H C C Cl + H2NR H C C NH R H Reagents: Conditions: 5. H alcohol room temperature H O 4. O H H C C O C C H + HCl H H H 3. + HCl + HCl H primary amine. room temperature. Reduction with lithium tetrahydridoaluminate (III) Acyl chlorides are reduced to primary alcohols by LiAlH 4 in the same way that the corresponding carboxylic acids are reduced. Arene 1. (i) Nitration Reaction type: Electrophilic substitution + HNO3 Reagents: NO2 + H2O concentrated nitric acid and concentrated sulphuric acid mixture. 11/16 565323042 Conditions: 55°C with constant agitation (reactants are immiscible). Knowledge of mechanism is required (not shown above). Above 60°C, a second nitration may take place at position 3. (ii) Reduction of nitro group to amine + 7 H+ NO2 + 3 SnCl2 + 12 Cl- 2- N+H3 + 3 SnCl6 + 2 H2O Reagents: Reaction conditions: Tin and concentrated hydrochloric acid These react together to form SnCl2 (with H2). Cold water bath to form the amine salt. The salt is then neutralised with concentrated NaOH (aq) to give the amine which is then steam distilled. N+H3 (aq) + NaOH (aq) NH2 + H2O (l) + NaCl (aq) (l) 2 Sulphonation Reaction type: Mechanism: Reagent: Substitution Electrophilic Fuming sulphuric acid (oleum) + SO3 3 Halogenation Reaction type: SO3H Electrophilic substitution + Br2 Reagents: Conditions: 4. Br + HBr R + HCl FeBr3 catalyst, bromine heat Alkylation (Friedel-Crafts reaction) Reaction type: Electrophilic substitution + RCl 12/16 565323042 Reagents: Conditions: chloroalkane and anhydrous AlCl3 (catalyst). heat under reflux. Attachment of an alkyl group activates the arene ring, making it susceptible to further alkylations. These occur at positions 2 and 4. 5. Conversion to an aromatic ketone (Friedel-Craft acylation) Reaction type: Electrophilic substitution O + O R C C R + HCl Cl Reagents: Conditions: 6 acyl chloride and anhydrous aluminium chloride catalyst. heat under reflux. Reduction + 3 H2 Reaction type: Conditions: addition Finely divided nickel, 150C Phenol 1 Reaction with alkali (but not with carbonates) Reaction type: Acid-base 2 O- Na+ + NaOH OH Conditions: Aqueous solution, room temperature Nitration Reaction type: Electrophilic substitution NO2 OH Reagent: Conditions: 3 + HNO3 OH 4 mole dm-3 (dilute) nitric acid room temperature Halogenation Reaction type: Electrophilic substitution 13/16 + H2O 565323042 Br + 3 Br2 OH Br OH + 3 HBr Br Conditions: Aqueous bromine, room temperature Amine 1. Conversion to amine salt Reaction type: Acid base R3N + HCl R3NH+ ClReagent: Conditions: 2. (R = alkyl, aryl or H group) dilute hydrochloric or sulphuric acid room temperature Reaction with transition metal ions Reaction type: complexation 4 R3N + Cu2+ [Cu(R3N)4]2+ (R = alkyl, aryl or H group) Reagent: Conditions: 3. Aqueous copper (II) sulphate solution Room temperature Conversion to N-substituted amide Reaction type: addition followed by elimination H O H O H C C Cl + H2NR H C C NH R H Reagents: H acyl chloride 14/16 + HCl 565323042 Conditions: 4. room temperature. Formation azo dye (from aromatic amines only) (i) Formation of diazonium salt NH2 + NaNO2 (aq) + 2 HCl (aq) N+ N Cl- (aq) Reagents: Conditions: (ii) + NaCl (aq) + 2 H2O sodium nitrite and dilute HCl (aq) less than 10°C Coupling of diazonium salt to aromatic amine or phenol N+ N Cl- (aq) N + NH2 NH2 + HCl N or 15/16 565323042 HO - N+ N Cl- (aq) O3S Na+ + HO - O3S N N + + HCl (aq) Na Nitrile 1 Conversion to carboxylic acid Reaction type: Hydrolysis Reaction conditions: Heat under reflux with NaOH, then neutralise with HCl (can also be hydrolysed with an acid catalyst) CH3CH2CN + OH- (aq) + H2O (l) → CH3CH2COO- (aq) + NH3 (aq) CH3CH2COO- (aq) + HCl (aq) → CH3CH2COOH (aq) + Cl- (aq) 2 Reduction to an amine Reagents: LiAlH4 in dry ether followed by H2O CH3CH2CN [ + 4 H] → CH3CH2CH2NH2 16/16