* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Sheet #12 Medicinal Plants
Survey
Document related concepts
Catalytic triad wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Biochemistry wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Transcript
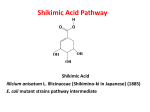
Medicinal plants Dr Fatima Afifi Lec no. : 12 lec pharm D : Lina Msllam The word ene means presence of unsaturated Last time we were talking about antibiotics lctone ring. originating from acetate malonate glycosidic derivatives which we divided to 3 groups: fused rings, macrolides and polyenes. Today we will continue with the last one of antimicrobial agents which is polyenes which is an antifungal agents. Last time we said that Griseofulvin which is a Polyenes are divided according to no. of conjugated double bonds, so we have : heptaene ,pentaene , hexa, tetra .. etc Amphotericin B : fused ring antibiotic possessing antifungal It has 7 conjugated double bonds so it’s a activities. heptaenes. The polyenes are similar in their structure to Notice no. of conjugated DB isn’t the same as no. lactone antibiotics for having a very huge lactone of DB - e.g: ring.(20-44 membered) Lactone ring usually has different types of Nystatin A have 6 DBs but it’s considered tetraene cuz it has only 4 DB as conjugated ones. substitutions; hydroxyl group mainly, but it could have glycosidic linkage, methyl group and it could have in its moieties an amino sugar. So these are the similarities between polyenes but what are the differences btween them ? They differ in the no. of unsaturation in their last So the largest no. of DB is necessary to classify these compounds. lactone ring. And that distinguishes them from Amphotercin B and cannabicidin are both macrolide antibiotics which don't have any hepatenes which are used for fungal infections, unsaturation in them. specially life threatening ones due to long term streptomyces are the usual producing organisms, there are around 200 hundred different types of polyenes isolated but only few of them could be use of tetracycline that cause elimination of normal flora, so there will be outgrowth of fungal infection . used as antifungal with restrictions due to high Candida Albican is the main m.o that causes this toxicity. infection. 1|Page Medicinal plants Dr Fatima Afifi Lec no. : 12 lec pharm D : Lina Msllam Amphotericin B and cannbicidin used to treat Antineoplastic Anthracyclin compounds have disseminated fungal infection by oral and IV. But broad spectrum of activity, and are used as standard or synthetic compounds, such as: the tetraenes like natamycin and nystatin A are used for other antifungal infections ;mainly skin infection and vaginal infections in topical route . Doxorubicin is one of the most used anti-tumors and have broad spectrum of activity. The anticancer potential is high in isolated and synthetic compounds. Nystatin A & natamycin are also used as eye drop to treat viral infecton in the eye. Erythromycin A is a macrolide antibiotic with uncommon sugar linkage. Adding a sugar linkage is the last rxn of microsynthesis. Amphotericin B have acteyl Co A and 15 malonyl Doxorubicin have high potency as newly CoA & 3 methylmalonyl Co A added with the synthesized cpd (compound) and is used as anti- presence of different methyl groups in the tumor. macrolactone ring. It’s also used as positive standard to check the The color of lactone compounds isn’t intensive potency of newly synthesized anticancerous due to high no. of polyenes (no. of conjugated agents. bonds), its usually in light yellow appearance with various shapes not a white crystalline structure.. Naturally antibiotics and anticancer products from plants origin are Tested in 6 different patterns for Another member of acetate malonate pathway their potency, if it was +ve result they will be are anticancer agents : compounds having isolated. polycyclic structure, they have antineoplastic activity and can be used to treat breast cancer ,childhood tumor, types of adenomas and cystic liver carcinoma. 2|Page Daunorubicin was isolated before and gained success in solid tumors , but few years later they discovered Doxorubicin which took lead due to higher potency. Medicinal plants Dr Fatima Afifi Doxorubicin is the oxidized form of Daunorubicin , Lec no. : 12 lec pharm D : Lina Msllam This was all about Anthracyclisn. These two cpds have identical structures only differing in their side chain, we have methyl group in Daunorubicin while there's hydroxymethyl group in doxorubicin. -NOW we move to shikmic acid pathway : Hybrid substances like c6- c3 cpds of phenyl propane derivatives have been isolated by Epirubicin: differ in the sugar moiety. Chinese scientists . At 1885 they discovered the shikimic acid The rest of cpds ( in slides) are similar only differ pathway : in methyl group or hydroxymethyl groups. Anticancers and antibiotics tend to produce Illicium anisatum: Shikimino is a Japanese name , analogues so they are more reasonably modified, it got its fame as a C6 –C3 acid material, that is one example: starting to make Tamiflu (drug for flu) which is They have uncommon glycosidic sugars Doxorubicin modified to Epirubicin which differ only in their stereochemistry of hydroxyl group of the sugar moiety at 4 prime. A Semi synthetic cpd of Daunorubicin is the highly demanded . So larger amounts are needed, and they did that by genetic engineering; enzymes were transferred to E.coli. This is an easy way to make it because these m.o Idarubicin, differing in presence of methoxy grp, are cheap and produce it quickly, its biochemistry daunorubicin was discovered in 1966, while is well studied, and known due to gene isolation dexarubicin1969. from plants and if we put it in larger mo than Regardless of their origin from plants or MO, they E.coli we get more amounts like in streptomyces. have important adverse effects which is unfortunately bone marrow suppression, which we can get 100 kilos !! causees new complications, and they are used in Shikimic acid is sometimes used as obligatory their IV route to be given in hospitals or for intermediate or raw material and therapeutic restricted studies in biochem labs. agent: They also cause GI disruptions, nausea and vomiting, lose of appetite and loss of hair. • Important for aromatic compounds production. 3|Page Medicinal plants Dr Fatima Afifi • Precursor of aromatic amino acids group shikmic acid which is mono unsaturated (Phenylalanine, Tyrosine, Tryptophan) cyclohexane carboxylic acid. biosynthesis in bacteria, fungi & higher plants. • Lec no. : 12 lec pharm D : Lina Msllam Building block for other compounds: certain alkaloids, coumarins, phenyl propanes, vol. oils,.. • Starting point for tannins in all plants • Provide protection of plants against micro-organisms . Phenylalanine is turned to tyrosine in our body by oxidation, tyrosine differs from phenylalanine by only having para-hydroxyl group Tryptophan is v. important and produces many huge compounds of alkaloids. Now we will discuss biosynthesis: Shikimic acid & acetate malonate produced from CHO have the same pathway for introducing terminal cpds. Many minor rxns which eliminate water can bring many new derivatives… carbonyl group can be easily reduced to hydroxyl group: e.g: Dehydro shikimc acid with mono unsaturated cyclohexane carboxylic acid can be reduced to its hydroxyl function. Quinic acid is also needed to precede synthesis as obligatory intermediate All of these rxns are reversible not one way and progress on the direction of demand. While shikimic acid is the Obligatory intermediate in these rxns we should be able to incorporate Refer to slide no. 3 in shikimic acid pathway radioactive shikimic acid to the pathway as a sign slides. that shikimic acid is involved. Shikimic acid forms Chorismic acid: at slide 4 shikimic acid is considered as a precursor for huge classes of biosynthesis , shikimic acid is obtained from 2 CHO cpds : Phosphoenol pyruvate 3 Carbon sugar with a 4 carbon sugar erytrhose -4- phosphate giving a heptulose to 3-dehydroquinic acid which is a carboxylic acid that is easily reduced to hydroxyl choriso (greek means splitting) Before breaking down chorismic acid have two options : 1- Form prephinic acid with its derivatives parahydroxy phenylpyruvate and phenylalanine 2- Anthranilic acid with its derivative tryptophan based on the indole nucleus. 4|Page Medicinal plants Dr Fatima Afifi Lec no. : 12 lec pharm D : Lina Msllam Tryptophan makes different alkaloids depending on the type of indole nucleus that’s present, such as indole acetic acid. Indole Acetic acid is a component of growth promoting hormones produce several synthetic analogues, like indole usinic acid which we will talk about in coumarins. From the prephinic acid pathway we have phenyl pyruvate which has ketoacids that are liable to amination rxns and Phenyl alanine that is obtained from glutamine, which can be converted to cinnamic acid, and it differs from tyrosine only in the presence of hydroxyl group in the para position. Converted to different aromatics depend on the presence of a.as. Tyrosine will be converted to para coumaric acids, while Phenylalanine is converted to Because there are many acids with processed c3c6 backbone, they are termed as cinnamic acid, para hydroxy Cinnamic acid & 3,4 dihydroxy cinnamic acid which is caffeic acid, they belong to the class of cinnamic acid regardless of substitution. Also due to the presence of many C6- C1 acids like hydroxybenzoic acid and methoxybenzoic acid, they are termed as benzoic acid regardless of substitution. These C6-C3 cpds can be transferred from cis to trans cinnamic acid easily, specially hydroxycinnamic acid, the nature favors the trans position in both coumarins and cinnamic acids. Another characteristic of c3-c6 cpds is: cinnamic acid through eliminating amino group by If there is one substituent of aromatic ring it is on transaminase rxn. para position to the side chain, if there are more They are precursors for different aromatic alkaloids. so this pathway provide cinnamic acid First substitution is usually in para position. than one it will be on different positions, if it's in orthro position we will discuss it later on in the coumarins. Biosynthetic pathways have different possibilities 1-Substitution in aromatic ring: we have parahydroxy, methoxy, 3,4-dihydroxy, (3,4,5trihydroxy) dreivatives. So we get cinnamic acids 5|Page Medicinal plants Dr Fatima Afifi Lec no. : 12 lec pharm D : Lina Msllam having different substitution pattern of their So two rxns happen simultaneously, we eliminate aromatic ring. the original carboxyl to get a new compounds. 2nd possibility : stage of oxidation of side chain: in cinnamic acid hydroxyl group can be obtained Therefore shikimic acid is used for making phenolic cpds. from their corresponding hydroxy-methyl We get phenolic substances regardless if the derivatives to obtain v. important class which is precursor have carboxyl group from the beginning alcohols: like polyphenyl alcohol. We modify or not, because it will be introduced to it. them by reduction to aldehyde, completely saturated CH3 or unsaturated, depending on the position of the side chain we get allyl side chain So shikimic acid is responsible for making phenolic cpds. or propane side chain as derivative of the acid so you have in your sheets names of different we have big no. of acids, alcohols …etc. substituted cinnamic acids so refer to them. 3rd possibility: Shortening of the side chain THE END Reducing no. of carbons from C6 –C3 C6- C1 C6. We can eliminate 2 carbons but from what origin? From the acetate we eliminate it by Beta oxidation .to get a resembling benzoic acid having the same substituents: parahydroxy, methoxygroup 3,4 dihydroxy or (3,4,5-trihydroxy). Shikimic acid responsible for production of phenolic substances, so we must have a phenyl hydroxy group. To get rid of carboxyl group, the compound should undergo decarboxylation. Decarboxylation of dihydroxy benzoic acid actually happens by Oxidative decarboxylation, 6|Page