* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Freezing Point of Water
Faster-than-light wikipedia , lookup
Modified Newtonian dynamics wikipedia , lookup
Wave packet wikipedia , lookup
Equations of motion wikipedia , lookup
Internal energy wikipedia , lookup
Hunting oscillation wikipedia , lookup
Photoelectric effect wikipedia , lookup
Centripetal force wikipedia , lookup
Electromagnetic spectrum wikipedia , lookup
Classical mechanics wikipedia , lookup
Thermal radiation wikipedia , lookup
Classical central-force problem wikipedia , lookup
Atomic theory wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
Work (physics) wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Heat transfer physics wikipedia , lookup
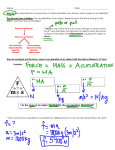
Spring Final Study Packet Measuring Heat 1. The freezing point of water is 0 °C and the boiling point is 100 °C. 2. The freezing point of water is 32 °F and the boiling point is 212 °F. 3. Temperature is the measurement of the average kinetic energy of a substance. 4. Energy is the ability to do work. Joule is the unit in which this is measured in. 5. Thermal Energy is the total energy stored in an object due to changes in temperature. That is, it’s the sum of all the kinetic and potential energy of the molecules of a substance. 6. Thermal Energy depends on three things: specific heat, mass and changes in temperature 7. Draw arrows to indicate where heat would move from one object to the other in the following diagrams Arrows between the air and the ice Write the correct term for each definition 8. the amount of heat energy needed to raise the temperature of a substance; specific to each material specific heat 9. the quantity of heat needed to increase the temperature of one pound of water by one degree Fahrenheit British thermal unit (BTU) 10. standard scientific temperature measurement, which includes absolute zero, the temperature at which all kinetic motion is said to stop kelvin 11. the measure of average kinetic energy in a material temperature 12. 100 divisions between the freezing point of water and the boiling point°C 13. 180 divisions between the freezing point of water and the boiling point °F 14. A sample of aluminum absorbs 330 calories of heat. If the temperature changes 15°C, how much aluminum is in the sample? (specific heat is 0.22 cal/g°C) 100 g How much energy is required to heat 35 grams of gold from 10°C to 50°C? (specific heat is 0.03 cal/g°C) 40 cal 15. Convert 79°C into Fahrenheit: 170 °F 16. Convert 583K into Celsius : 310. °C 17. Convert 59°F into Celsius: 15°C Spring Final Study Packet Heat Transfer 1. Conduction is the transfer of energy direct contact. 2. Convection is the transfer of energy through a fluid. 3. Radiation is the transfer of energy through a vacuum. 4. Any material that can flow is a fluid. 5. The method used by scientists to determine the amount of energy in food is called calorimetry. 6. From the electromagnetic spectrum (light), what type of wave is heat? infrared 7. From the electromagnetic spectrum (light), what color is hottest? violet 8. Heating an object does what to the volume of it? increases 9. What type of heat transfer is this? conduction 10. What is the name of the object below? Convection box 11. What type of heat transfer is this? convection 12. Draw an arrow to indicate the direction of the smoke of the punk in the diagram. 13. A insulator is an object or material that does not allow heat to pass through easily. An example would be rubber, wood, plastic, glass, air etc. Identify the following as an example of conduction, convection or radiation: Light bulb radiation Aluminum transfer bar in calorimetry lab conduction Spoon in hot soup conduction Hot air off the top of a candle convection Wind convection Curling iron conduction Calorimetry A nutritionist wishes to know the energy value for a piece of food. She uses a calorimeter in which she places 200.0g of water. The change in temperature in the water after the food has been completely combusted is 15.0 °C. How much heat energy was stored in the food? HINT: Look at how much heat energy was gained by the water. 3000 cal = 3.0 x 10 3 cal Spring Final Study Packet Nuclear Chemistry Consider this isotope symbol: 15N7 How many protons? 7 How many neutrons? 8 What is the mass number? 15 What is the atomic number? 7 2. From which part of the atom do radioactive emissions originate? nucleus 3. What effect does the emission of an alpha particle have on the mass number and the atomic number of the original isotope? Mass drops by four, proton number by 2 4. What effect does the emission of a beta particle have on the mass number and the atomic number of the original isotope? Mass number stays the same proton number goes up one 5. How is a positron formed? Proton splits into a neutron and positron; positron is emitted and neutron stays 6. What effect does the emission of positron have on the mass number and the atomic number of the original isotope? _ Mass number stays the same and proton number goes down one 7. What particle has a mass of 1 amu and has no charge? Neutron 8. What is the nuclear symbol of this particle?1 0 n 9. What particle has a mass of 1 amu and has a 1+ charge? Proton 10. What is the nuclear symbol of this particle? 1 1 p 11. All nuclei with an atomic number above 92 are radioactive 12. Define half-life. Amount of time it takes for half of the sample to decay 13. Define transmutation. Changing of one element into another 14. The number of protons in the nucleus of an atom must change for transmutation to occur. 15. If a large nucleus is bombarded with neutrons and two smaller nuclei are formed, the process is called fission. 16. If two nuclei combine to form a heavier nucleus, the process is called fusion 17. Show the reaction that occurs when the following isotopes emit an alpha particle. 238 Np 4He 93 + 234 Pa 2 91 18. Show the reaction that occurs when the following isotopes emit a beta particle. 13C 0 6 e 13 + -1 N 7 19. Show the reaction that occurs when the following isotopes emit a positron. 222 87 Fr 222 86 Rn + 0 +1 e Spring Final Study Packet Motion 1. A person walks 20 meters down a hallway, turns around and walks back 5 meters. What is the displacement? 15 m down thehall What is the distance traveled? 25m 2. Use the space below to draw out the following pattern: 3. a. 2.0 cm North from 0,0 (label this initial) b. 1.0 cm East c. 2.0 cm North d. 3.0 cm East (label this final) 4. What is the total distance traveled? 6 cm 5. What is the displacement? 4.5 cm NE 6. Calculate the displacement using Pythagorean theorem 4.5 cm NE 7. According to the chart to the right, what is the average speed of the object? 9m / 3s = 3 m/s Time (s) 0 1 2 3 Distance (m) 0 3 6 9 8. Is this object accelerating? No, it is traveling at a constant speed each second 9. While traveling along the highway a driver slows from 24m/s to 15 m/s in 12 seconds, what is the driver’s acceleration? 15 m/s – 24 m/s = - 0.75 m/s2 12 s 10. A bicycle rider travels 60.0 km in 3.5 hours. What is the cyclist’s average speed? 60.0 km / 3.5 hours = 17 km/hr 11. How much time would it take for the sound of thunder to travel 1,500 meters if sound travels at the speed of 330m/s? 330 m/s = 1500 / x (4.5s) Spring Final Study Packet 12. A snail can move approximately 0.30 meters per minute. How many meters can the snail travel in 15 minutes? 0.30 m/min = x/15 min (4.5 m) 13. An ice cream truck is moving along at 20m/s. It then speeds up to 28 m/s in 5 seconds. What is the acceleration of the truck? 28 m/s – 20 m/s = 1.6 m/s2 rounded to 2 m/s2 for sig figs 5s 14. If a rocket in space is traveling at a constant velocity of 9.8 m/s and then uses its propulsion system to accelerate to 10. m/s during a 3.0 minute burn, what is the acceleration of the rocket? 10.m/s – 9.8 m/s = 0.067 m/s/min 3.0 min Write a description of the motion in the graphs below: Constant velocity Constant velocity Moving backward at Constant velocity Newton’s Laws 1. Mass never changes but weight is different with gravity differences. 2. What is the weight of a 2.0kg mass? F = ma; F = 2.0kg * 10. m/s2; F = 20. N 3. What is the mass of a 20. N object on Earth? F = ma; 20.N = m * 10. m/s2 m = 2.0 kg 4. Friction always opposes motion. 5. What is the net force if an airplane has a thrust force of 600N and an air friction of 200N? 600N – 200N = 400 N 6. If the net force is 20N and the sliding friction is 5N, with what force is a box being pushed across the floor? 20N = F - 5N; F = 15N 7. If a car has a forward motion of 45N and the rolling friction is -45N, what is the acceleration of the car? 0N; When the net force is zero the object has constant velocity therefore, no acceleration. Spring Final Study Packet Newton’s 1st Law of Motion 8. The first law is also called the law of inertia. 9. This property is directly related to mass. 10. Rank the following by order of most inertia (1) to least inertia (5) a. baseball b. bowling ball c. feather d. car e. marble 3 2 5 1 4 Newton’s 2nd Law of Motion 11. Mass resists acceleration 12. Forces cause acceleration 13. Acceleration equals the ratio of force over mass 14. A heavier object accelerates slower (less) than a lighter object. 15. A net force of 25 is applied to an object and it accelerates 10. m/s2, what is the mass of the object? F = ma; 25N = m * 10. m/s2; m = 2.5 kg Newton’s 3rd Law of Motion Identify the action reaction pairs below: A ball hitting a wall. A bug hitting a windshield. Action: The ball hits the wall Action: A bug hits a windshield Reacton: The wall hits the ball Reaction: The windshield hits the bug. Identify which of Newton’s 3 laws applies Pushing a cart down the hall, when you try to turn it, it tries to go straight. Newton’s First Law More acceleration takes more force Newton’s Second Law When you push your knuckles into a table, it hurts your knuckles. Newton’s Third Law A ball thrown into a wall bounces back. Newton’s Third Law Work, Power and Energy W = Fd P = W / t KE = ½ mv2 PE = mgh 1. If a lawnmower is pushed 8.5 meters and 2550 Joules of work is performed, how much force is needed to push the lawnmower? a. 0.0033 N c. 19 000 N b. 300 N d. 20 000 N 2. A student climbing the stairs covers 6.4 m. The student weighs 500 N. How much work did the girl do in climbing the stairs? a. 3000 J c. 0.1 J b. 70 J d. 5 J 3. In 20.0 seconds, a large crate is pushed up a 9.0 meter plank. A force of 25 Newtons is required. How much power is used? a. 11 W c. 220 W b. 9.0 W d. 180 W Spring Final Study Packet 4. A boy runs 100 meters in 7 seconds. He is 600 N. How much power does he expend? a. 60 000 W c. 9000 W b. 4000 W d. 40 W 5. Calculate the kinetic energy of a roller coaster car with a mass of 20.0 kg going a speed of 4.0 m/s. a. 800 J c. 160 J b. 5.0 J d. 40 J 6. If a ball is held from a height of 50 m that weighs 0.02 kg, how much PE does it have? a. 10 J c. 90 J b. 1 J d. 2000 J Fill in the Chart a c d b e e b d c a Harmonic Motion and Waves 1. What is the formula for calculating wave speed? S = f λ (frequency x wavelength) 2. If the speed of a wave is 120 m/s and the wavelength is 60.0 m, what would be the frequency of the wave? 2.0 Hz 3. Which wave type moves perpendicular to wave direction? transverse 4. Which wave type moves parallel to wave direction? Compressional (longitudinal) Label all of the following parts. A - transverse B – wavelength C – Crest D – trough E - amplitude AB – longitudinal AC – wavelength AD – compression AE - rarefaction Spring Final Study Packet 5. What type of matter is most efficient at transmitting sound waves? Sound travels faster through solids (more dense materials) 6. Define natural frequency the frequency at which objects will vibrate when disturbed 7. If the fundamental frequency is 20Hz, what is the frequency of the 5th harmonic? 100Hz (harmonic x natural frequency) 8. What is the harmonic of the diagram to the right? 3 9. What would be an example of harmonic motion? Anything that repeats Sound and Light 1. Sound is a compressional wave, which moves parallel to the medium it is traveling through. 2. Sound travels best through dense material (solids) 3. What is the wavelength of a sound wave with a frequency of 100 Hz? (speed of sound is 340 m/s) 3.5 m 4. If your echo takes 15 seconds to get back to you, how far away was the object it bounced off of? S = d/s; 340 m/s = d/15 s; 5100m 5. True or False: The frequency of sound has no effect on how loud we hear the sound. Frequency is pitch amplitude is volume 6. What must happen to the electron in order for an atom to emit light? a. Move from a low energy level to a high energy level b. Stay in a high energy level c. Move from a high energy level to a low energy level d. Stay in a low energy level 7. What are the three primary colors of light? Red, Green, Blue (RGB) 8. What are the three primary colors of pigments? Cyan, magenta, yellow (CMY) 9. What does a piece of blue cloth do to the colors in white light that fall upon it a. It absorbs blue light and reflects all the rest of the colors to our eyes b. It absorbs all the colors except blue and reflects only blue light to our eyes c. It absorbs all of the colors in white light d. It absorbs none of the colors in white light 10. Most of what we see is because of reflected light. 11. List the types of electromagnetic waves from lowest energy to highest energy: radio, microwave, infrared, visible, UV, x-ray, gamma