* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Clinical Genetics- BH6N-601A
Survey
Document related concepts
Transcript
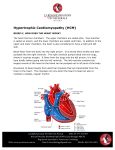
Insurance Company Name Address City, State Date of claim Re: Ambry Genetics Corporation, Letter of Medical Necessity (LMN) for HCM Panel Patient First, Last Name DOB ID Number Dear Medical Director, This letter is in regards to my patient and your subscriber, First, Last Name to request full coverage for DNA sequence analysis for a panel of 31 genes when mutated are known to be associated with hypertrophic cardiomyopathy (HCM)to be performed by Ambry Genetics Corporation (TIN 33-0892453 / NPI 1861568784), a CAP-approved and CLIA-certified laboratory located at 15 Argonaut, Aliso Viejo, CA 92656. Referenced genes include: ACTC1, ACTN2, ANKRD1, CALR3, CAV3, CSRP3, DES, FXN, GLA, JPH2, LAMP2, MYBPC3, MYH6, MYH7, MYL2, MYL3, MYOM1, MYOZ2, NEXN, PLN, PRKAG2, PTPN11, RAF1, TCAP, TNNC1, TNNI3, TNNT2, TPM1, TTN, TTR and VCL. Hypertrophic cardiomyopathy occurs with a prevalence of 1 in 500 and is suspected in individuals whose personal or family histories include any of the following: Structural changes of the heart including thickening of the left ventricular wall Severe fatique (present in 20%) Arrhythmias Exercise fatique and syncope Congestive heart failure, embolic stroke and sudden cardiac death (present in 10%) As such, First, Last Name personal and/or family history(ies) are suggestive of hypertrophic cardiomyopathy susceptibility. Based on my evaluation and review of the available literature, Ambry Genetics’ 31 gene panel testing for hypertrophic cardiomyopathy is crucial in order to establish/confirm a genetic diagnosis and in guiding appropriate and immediate medical management. A positive genetic test result can provide the following benefits to this patient: Appropriate management and other treatment guidance Predict disease prognosis and risk for secondary findings Help identify the cause for HCM Allow specific gene analysis for family members when a familial mutation has been identified Help identify family members who are not at an increased risk to develop cardiomyopathy Genetic testing will be performed through Ambry Genetics Corporation given their longstanding experience with next-generation sequencing, consistent variant analysis, detailed results reporting and continuous support from highly trained medical directors and genetic counselors. Ambry Genetics offers the most comprehensive genetic testing for HCM by sequencing the entire coding region of all clinically available genes responsible for HCM. At least 40 – 80% of all cases of hypertrophic cardiomyopathy are estimated to be due to the 31 genes included in the HCM Panel. The majority of the remaining 20 -60% of inherited HCM is thought to be due to a mutation in yet unidentified genes and clinical testing is not available. I recommend that you support this request for coverage of diagnostic genetic testing for HCM for my patient. Genetic testing can take up to four months to complete and the laboratory will not bill until testing is complete. Therefore, we are requesting that the authorization be valid for 6 months. SUMMARY OF DIAGNOSIS - ICD-9 CODES (INPUT ALL THAT APPLY) – (USE V CODES FOR SECONDARY DX) 359.10 Hereditary progressive muscular 428.0 Congestive heart failure dystrophy 425.11 Hypertrophic obstructive cardiomyopathy 428.1 Left heart failure 759.89 Noonan syndrome 441.03 Thoracic aortic dissection 780.2 Syncope and collapse 441.9 Aortic aneurysm of uns site 794.31 Abnormal EKG 425.18 Hypertrophic non-obstructive cardiomyopathy 425.4 759.82 Marfan syndrome 745.4 Cardiomyopathy, other primary 746.3 w/o rupture V12.53 Hx. sudden cardiac arrest Ventricular septal defect V18.9 Genetic disease carrier Aortic stenosis, congenital V82.71 Scr for genetic disease carrier status 425.8 Syndromic cardiomyopathy 746.89 Brugada syndrome 426.82 Long QTc/Long QT syndrome 746.9 Congenital heart disease (NOS) Others _______________________________ _______________________________ Thank you for your time and please don’t hesitate to contact me with any questions. Sincerely, Ordering Clinician Signature ________________________________ Date ______________ (MD/DO, Clinical Nurse Specialist, Nurse-Midwives, Nurse Practitioner, Physician Assistant, Genetic Counselor*) *Authorized clinician requirements vary by state