* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download EMERGING TREATMENT ISSUES IN MDR-TB
Survey
Document related concepts
Transcript
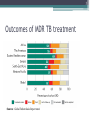
Treatment for Multi-drug Resistant TB STREAM Clinical Trial [INSERT NAME OF PRESENTER] 2 Status of MDR-TB world-wide Burden of disease • In 2015, of the estimated 580,000 people eligible for MDR-TB treatment, only 125 000 (~ 1 in 5) were started on treatment • Outcomes of MDR/RR-TB patients in the 2013 cohort: ▫ ▫ ▫ ▫ ▫ 52% were successfully treated 17% died 15% were lost to follow-up 9% were determined to be treatment failure 7% had no outcome information 3 Outcomes of MDR TB treatment Source: Global Tuberculosis Report 2016 4 Where does MDR TB occur? 5 Treatment of drug sensitive TB • Two main drugs for treatment of DS TB ▫ Isoniazid ▫ Rifampicin • Treatment taken 6 months by mouth • Treatment very successful ▫ >90% cure for patients who take their medication • If resistance to rifampicin occurs treatment ▫ takes much longer ▫ more side effects does not work as well 6 How does resistance to TB medicines happen? • Acquired resistance happens when ▫ Patients with drug sensitive TB can’t or don’t take medications as required Miss doses or not given the correct doses to take Pharmacy runs out of medicines Get side effects and stop treatment • Transmitted resistance happens when ▫ Patients get infected with resistant TB TB organism that infects the patient is resistant to medication from the start of treatment 7 What if TB resistance exists? • Rifampicin is the best currently available TB medicine • If resistance to Rifampicin exists, more medications have to be given: ▫ not as strong ▫ worse side effects • Patients have to have injections for 6 months • Treatment less effective ▫ Only ~50% of patients are cured 8 Definitions of TB Drug Resistance Drug Sensitive Multi-drug resistant Pre-XDR Rifampicin Rifampicin Rifampicin Rifampicin Isoniazid Isoniazid Isoniazid Isoniazid Fluoroquinolne Fluoroquinolne Extensively drug resistant or Amikacin or kanamycin or capreomycin Amikacin or kanamycin or capreomycin 9 How is DR TB treated? Introdion Objectif Méthodes Conclusion 10 Conventional/longer regimens for MDR-TB • Usually between 20-24 months, with 2 phases • Intensive phase ▫ ▫ ▫ ▫ ▫ ▫ Quinolone (moxifloxacin) Injectable agent (amikacin, kanamycin or capreomycin) PZA Ethionamide Terizidone/cycloserine Ethambutol (depends on local prevalence or resistance) ▫ ▫ ▫ ▫ ▫ Injectable agent (amikacin, kanamycin or capreomycin) PZA Ethionamide Terizidone/cycloserine Ethambutol (depends on local prevalence or resistance) • Continuation phase 11 New WHO recommendation for shorter regimens - 2016 ▫ For most patients with MDR-TB, a shorter regimen (9-12 months) can be used instead of a longer, conventional regimen ▫ But: WHO’s recommendation acknowledges that additional evidence is required 12 Emerging evidence for new regimens • Results for six groups of patients with MDR-TB in Bangladesh who received a shortened regimen indicated there might be better treatment options for MDR-TB, even without new drugs ▫ Van Deun A, Maug AKJ, Salim MAH, Das PK, Sarker MR, Daru P, et al. Short, Highly Effective, and Inexpensive Standardized Treatment of Multidrug-resistant Tuberculosis. Am J Respir Crit Care Med. 2010; 182(5): 684-92. 13 Results of 9-month regimen–Bangladesh Published cohort (206 pts) Cure Cohort update (515 pts) 82.5% 81.2% Completion 5.3% 3.3% Default 5.8% 7.8% Death 5.3% 5.6% Failure 0.5% 1.4% Relapse 0.5% 0.8% Overall success rate: Overall success rate: 87.9% (95% CI 82.7, 92.6) 84.5% (95% CI 0.81, 0.88) Introdion Objectif Conclusion Méthodes Am J Respir Crit Care Med Vol 182. 684–692, 2010 Aung et all, IJTLD 18(10):1180–1187, 2014 Bangladesh regimen–Intensive Phase • Quinolone (moxifloxacin/ gatifloxacin) • Injectable agent (Amikacin, kanamycin or capreomycin) • PZA • Ethionamide/prothionamide • High dose INH • Clofazamine • Ethambutol 15 Bangladesh regimen–Continuation Phase • • • • • Quinolone (moxifloxacin/gatifloxacin) PZA Clofazamine Ethambutol No injectable agent 16 Conclusions • Drug resistant TB affects > half a million people worldwide • Only half of those who are treated for Drug resistant TB are successfully treated • Until recently, treatment for Drug Resistant TB was based on very low quality evidence • Promising new treatment regimens for MDR-TB are emerging: ▫ ▫ ▫ ▫ Better treatment outcomes Shorter Still includes injectable agents Do not yet incorporate new drugs • However, there continues to be an urgent need for research The STREAM Trial [INSERT NAME OF PRESENTER] 18 Building evidence for better MDR-TB regimens Cohort studies Randomized trials • • • • • • • STREAM Cameroon Benin Niger Swaziland Other African countries Uzbekistan 19 STREAM objectives vs. Conventional regimen (20-24 months) vs. Shorter regimen (6 months) Bangladesh regimen w/ Moxi (9 months) vs. All oral regimen (9 months) 20 STREAM Stage 1 • Control regimen (A) STREAM Stage 1 sites locally used WHOrecommended regimen • Study regimen (B) similar to Bangladesh shorter regimen but high-dose moxifloxacin replaces high-dose gatifloxacin Sites: Ethiopia (2), South Africa (3), Vietnam and Mongolia (1) 21 The study regimen (B) – 9 months Weeks Drug Kanamycin* Isoniazid (H) Prothionamide Clofazimine Moxifloxacin Ethambutol Pyrazinamide 2000 mg Drug doses by weight group < 33 kg 1 - 16 1 - 16 1 - 16 1 - 40 1 - 40 1 - 40 1 - 40 33 - 50 kg > 50 kg 15 mg per kilogramme body weight 300 mg 400 mg 600 mg 250 mg 500 mg 750 mg 50 mg 100 mg 100 mg 400 mg 600 mg 800 mg 800 mg 800 mg 1200 mg 1000 mg 1500 mg • Kanamycin 3 times/week after week 12 The intensive phase may be extended by 4 or 8 weeks if smear conversion has not occurred by 16 or 20 weeks 22 STREAM Stage 1 study population • Adults who have given consent • Smear-positive pulmonary tuberculosis or, if HIV positive, may be smear negative • Resistance to rifampicin • No resistance to fluoroquinolone or 2nd-line injectables • No pre-existing QT prolongation >500msec • If pre-menopausal woman, not pregnant or breast feeding and agrees to use effective barrier contraception/IUCD during treatment 23 STREAM Stage 1 status • Enrolment started July 2012 • 424 patients enrolled ▫ Target reached and exceeded • Intake closed June 30th 2015 • Retention rates are high • Last patient visit expected Q4 2017 • Results from Stage 1 expected Q1/2 2018 24 STREAM Stage 2 vs. Conventional regimen (20-24 months) vs. Shorter regimen (6 months) Bangladesh regimen w/ Moxi (9 months) vs. All oral regimen (9 months) 25 STREAM Stage 2 history • After provisional licensing of first new TB drug for ~50 years (bedaquiline) in 2013, STREAM trial sponsor asked: ▫ Is it possible to add regimens to the STREAM trial? ▫ What would be the appropriate regimen(s) to add? • After extensive discussions with experts it was agreed that the primary interest to patients and programmes would be: ▫ A fully oral regimen (no kanamycin) and/or ▫ A shorter/simpler regimen 26 STREAM Stage 2 regimens STREAM Stage 2 patients take one of four regimens: A: the locally used WHO approved MDR-TB regimen B: the modified 9-month Bangladesh regimen studied in Stage 1 C: a fully oral 9-month regimen D: a six-month regimen Both regimens C and D will contain bedaquiline throughout. 27 STREAM Stage 2 regimens 28 STREAM Stage 2 objectives • Assess whether Regimen C, the fully oral regimen, is as effective as Regimen B at 76 weeks (18 months) • Assess whether Regimen D, the 6-month regimen, is as effective as Regimen B at 76 weeks (18 months) 29 STREAM Stage 2 status • Enrolment began in March 2016 STREAM Stage 2 sites ▫ >70 patients enrolled • Currently enrolling patients in Ethiopia, Mongolia, South Africa and Moldova • Trial planned for 9 countries and 15+ sites Current sites: Ethiopia (2), South Africa (3), Mongolia (1), Moldova (1) 30 STREAM Stage 2 study population • The same criteria as Stage 2 with ▫ Additional safety criteria for liver, kidney, pancreatic and electrolytes function ▫ Increased focus on excluding risk factors for cardiac arrhythmias ▫ A chest X-ray compatible with a diagnosis of pulmonary TB ▫ HIV infected participants must CD4 count more than 50 cells/mm3 31 STREAM Stage 2 timeline • Plan to complete enrolment in less than 3 years • Last patient enrolled Q4 of 2018 • Last patient reaches 18 months postrandomisation Q2 of 2020 • Last patient completes long term follow-up Q2 of 2021 32 STREAM Partners Funder: USAID Funder: Janssen Pharmaceuticals (Stage 2 only) Sponsor: Microbiology: Institute of Tropical Medicine, Antwerp Design, Management, Analysis Trial sites Impact Assessment: Liverpool School of Tropical Medicine 33 Conclusions • Drug resistant TB affects > half a million people worldwide • Only half of those who are treated for drug resistant TB are successfully treated • Until recently, treatment for drug resistant TB was based on very low quality evidence • Promising new treatment regimens for MDR-TB are emerging: ▫ ▫ ▫ ▫ Better treatment outcomes Shorter Still includes injectable agents Do not yet incorporate new drugs • However, there continues to be an urgent need for research 34 Conclusions • STREAM aims to improve the evidence base for better MDR-TB treatment regimens ▫ Shorter regimens (Stage 1 and 2) ▫ All-oral regimen (Stage 2) ▫ New drugs (Stage 2) • STREAM uses a randomized control trial design to strengthen quality of evidence • Results from Stage 1 expected shortly • Stage 2 still scaling up ▫ Results not expected until after 2021 Thank you