* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download micro notes chpt. 8
Biosynthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Mitochondrion wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
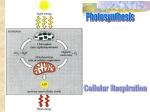
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Micro Notes CHAPTER 8 1. a. Enzymes, which are typically proteins, catalyze chemical reactions. The polypeptide chain, when properly configured in its tertiary structure, will have an active site or substrate-binding domain that is involved in catalysis. Conformational changes occur during catalysis and return the enzyme to its original configuration after release of product(s) so that it is able to bind more substrate. An apoenzyme is an enzyme that requires a cofactor or coenzyme to achieve the proper substrate binding conformation or to undergo catalytic changes in conformation. b. Figures 8.5 and 8.6 show the interaction of the substrate (and cofactor) with a holoenzyme and the products formed. In general, the substrate fits into the active site of the enzyme like a “lockand-key.” A cofactor or coenzyme might assist in the proper fit. Catalysis involves conformational changes in the enzyme, which then result in release of products. Enzymes may be synthetic (combine substrates), degradative (break a bond in a substrate), or function in the transfer of a chemical group from one substrate to another. 2. Cofactors, including metal ions such as iron, copper, and magnesium, activate enzymes, help with substrate binding to the active site, and participate directly in catalysis. Cofactors called coenzymes are organic compounds, such as vitamins, that work in conjunction with the apoenzyme to alter a substrate, typically by acting as a transient carrier of a chemical group between substrates. 3. a. Phosphoglucoisomerase converts glucose-6-phosphate to its isomer fructose-6-phosphate. If fructose-6-P is not readily phosphorylated to form F-1, 6-P, then phosphoglucoisomerase will be inhibited by feedback from its product. Pyruvate kinase transfers the high-energy phosphate bond from phosphoenolpyruvic acid to ADP with the resulting products being pyruvic acid and ATP. If pyruvic acid does not enter the TCA cycle or fermentation, it attenuates pyruvate synthase through feedback inhibition. Likewise, a lack of ADP or excess of ATP will inhibit the enzyme. b. These regulators may be placed in Figure 8.18 by drawing feedback arrows to indicate the nature of the inhibition (e.g. an arrow from F-6-P to the blue arrow indicating catalysis in step 2 by phosphoglucoisomerase). 4. a. Oxidation can occur in the absence of oxygen when an electron carrier such as NAD acts in a redox reaction. b. In this equation, glucose is oxidized (loses electrons) and oxygen is reduced (gains electrons); oxygen is an oxidizing agent because it accepts electrons and glucose is a reducing agent because it gives up electrons. (Remember that the electrons are accompanied by hydrogens). 5. a. NAD is oxidized and NADH is reduced. b. FADH2 is reduced and FAD is oxidized. c. Lactic acid is reduced and pyruvic acid is oxidized. d. NO3- is oxidized and NO2- is reduced. e. Ethanol is reduced and acetaldehyde is oxidized. 6. Chapter 8 a. ATP is an energy carrier, holding it in its high-energy phosphate bonds, which transfers energy to initiate glycolysis. It is also formed by substrate-level and oxidative phosphorylations of ADP during metabolism. It also transfers energy to initiate numerous chemical reactions in the cell. NAD is an electron carrier and is reduced to NADH when it picks up electrons from glycolysis and the TCA cycle. The energy of the electrons is transferred to the electron transport system to power the oxidative phosphorylation of ADP to ATP. b. ATP carries energy in the high-energy bonds between phosphate groups. The nicotinamide group of NAD is suited to be reduced or to accept electrons. 7. a. Anabolism includes synthetic reactions that require energy. Catabolism includes degradative reactions that release energy. Anabolic and catabolic reactions are typically coupled, where energy released from one is used to drive the other. b. When the energy carrier ATP is hydrolyzed, it releases energy from the phosphate bond and yields ADP and inorganic phosphate. ATP is regenerated when ADP is phosphorylated during metabolism and energy is again stored in the phosphate bond. c. Glycolysis is the breakdown of glucose to yield pyruvic acid, ATP, and NADH. Pyruvic acid will enter the TCA cycle and yield an abundance of ATP if oxygen is present. If no oxygen is available, pyruvic acid will enter the fermentation pathway and be converted to acids, alcohols, and/or gases but will not yield any ATP, but rather only NADH. d. The electron transport system accepts electrons from NADH produced during glycolysis and the TCA cycle. The energy is used to create a proton gradient across the membrane, which is then used to power a proton-driven ATP synthase that produces ATP by oxidative phosphorylation of ADP. 8. a. As NADH or FADH2 donate electrons during electron transport, the final electron acceptor drives the progression of redox reactions down the transport chain and eventually leads to the reduction of the final electron acceptor. Without the final electron acceptor, the reactions do not proceed forward. b. The final electron acceptor(s) in aerobic metabolism is oxygen; in anaerobic metabolism are oxygen-containing ions (e.g. nitrate); and in fermentation are organic compounds (e.g. acetaldehyde). c. In aerobic respiration, oxygen is the final electron/H+ acceptor. An essential enzyme, cytochrome oxidase (aa3), stationed in the crista membrane of the mitochondrion of eukaryotes and in the cell membrane of some bacteria, is adapted to bringing the reactants together. It accepts the electron pairs from cytochrome c, picks up H+ from the nearby solution, and completes the bonding between the electrons, hydrogens, and oxygen. The final product is H2O. 9. Substrate-level phosphorylation, which occurs in the cytoplasm, involves the enzymatic transfer of phosphate from metabolic intermediates (e.g. phosphoenolpyruvic acid) to ADP to yield ATP. Oxidative phosphorylation utilizes the energy from a proton gradient to power a membraneembedded ATP synthase to phosophorylate ADP with inorganic phosphate to yield ATP. 10. Glycolysis occurs in the cytoplasm of both prokaryotic and eukaryotic cells. In eukaryotic cells, the TCA cycle occurs in the mitochondrion and electron transport occurs in the mitochondrial inner membrane. In prokaryotic cells, the TCA cycle occurs in the cytoplasm and electron transport occurs in the cell membrane. 11. a. To initially jump-start glycolysis, ATP is used to phosphorylate glucose to produce glucose-6-P, which is then converted to fructose-6-P and phosphorylated with use of ATP to produce fructose1, 6-P (2 ATP consumed per glucose). F-1, 6-P is split into 2 molecules of glyceraldehyde-3-P, which is then converted to disphophoglyceric acid. ATP is then produced by substrate-level Writing to Learn Questions Page 2 of 4 Chapter 8 phosphorylation of ADP to yield 3-phosphoglyceric acid, which is then converted to 2phosphoglyceric acid and then phosphoenolpyruvic acid (2 ATP produced per glucose). ATP is produced by substrate-level phosphorylation of ADP to yield pyruvic acid (2 ATP produced per glucose). The net yield of ATP per glucose is 2. b. NADH is produced during glycolysis and the TC cycle. NADH, in aerobes, is used to donate electrons to the electron transport chain for the purpose of generating ATP. c. NADH, in fermentative organisms, is also used to donate electrons directly to organic compounds that serve as the final electron acceptors. This process generates no additional ATP but it does drive an increased rate of glycolysis in which more ATP can be generated. 12. a. In the TCA cycle, ATP is produced by substrate-level phosphorylation of ADP during the conversion of succinyl-CoA to succinic acid. Additional ATP molecules are produced from electron carriers from the TCA cycle and oxidative phosphorylation. b. 38 molecules of ATP (40 produced and 2 used) are formed from each glucose molecule. c. Eukaryotes produce 38 ATP molecules from the metabolism of one molecule of glucose, but this number can vary amongst prokaryotic microbes (36-38 ATP). Factors that contribute to this difference are structural differences between gram-positive and gram-negative bacterial cell membranes (e.g. periplasm) and the variety of electron transport cytochromes as compared to eukaryotes. 13. The sources of oxygen in anaerobic bacteria include nitrates, carbonates, and sulfates. 14. a. Chemiosmosis involves the creation of a proton gradient across a membrane by the electron transport system and use of the gradient and proton diffusion (proton motive force or PMF) to power a membrane-embedded ATP synthase, which converts ADP and inorganic phosphate to ATP by oxidative phosphorylation. b. ATP synthase allows proton diffusion through one subunit, which then causes a conformational change that allows the other subunits to act upon ADP and inorganic phosphate. 15. Aerobic and anaerobic respirations differ in their requirements for oxygen and alternative terminal electron acceptors. Per glucose molecule, aerobic respiration produces 38 ATP, whereas anaerobic respiration produces much less. 16. For each glucose molecule, 6 oxygen molecules are consumed and 6 carbon dioxide and 6 water molecules are produced. 10 NADH and 2 FADH2 molecules are produced; however, they are consumed by electron transport and chemiosmosis to produce 34 ATP to yield a total of 38 ATP from the entire pathway. 17. a. ATP synthase; RNase b. hexokinase c. lactic dehydrogenase d. nitrate reductase 18. a. Light dependent: Oxygen, NADPH, and ATP Light independent: Glucose Writing to Learn Questions Page 3 of 4 Chapter 8 b. Water is consumed during the light reaction while carbon dioxide is consumed during the dark reaction. c. Photosystems serve as the site of photosynthesis, while chlorophyll molecules (contained within the photosystem) release electrons as they absorb light and eventually form ATP and NADPH. d. ATP and NADPH are fed into the stroma of the chloroplast and enter the Calvin cycle, eventually producing glucose. e. Oxygenic photosynthesis uses H2O as a source of electrons and produces oxygen as a byproduct. Anoxygenic photosynthesis employs a cyclic photosystem that uses H2 and H2S as a source of electrons and consequently produces no oxygen. Writing to Learn Questions Page 4 of 4