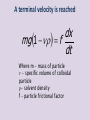* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 05.Kinetic Optical Properties of Colloids
Survey
Document related concepts
Bose–Einstein condensate wikipedia , lookup
Gibbs paradox wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Van der Waals equation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Cross section (physics) wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Freeze-casting wikipedia , lookup
Electron scattering wikipedia , lookup
Transcript
Kinetic/Optical Properties of Colloids PhD Halina Falfushynska Motion • Thermal motion – Brownian Motion on the microscopic scale – Diffusion and translation on the macroscopic scale • Techniques for measuring colloidal sizes – Sedimentation (under gravitational or applied field) – Colligative Properties – Scattering techniques Robert Brown (1827) vsed Brownian motion Peclet Mainz-02-11-04 George Gabriel Stokes (1851) mg Albert Einstein (1905) D advection Rv sed mgR Pe diffusion D kBT kT 3 Sedimentation Potential Sedimentation Of A Single Particle Generates a Potential Stokes Law Stokes' law, for the frictional force – also called drag force – exerted on spherical objects with very small Reynolds numbers (e.g., very small particles) in a continuous viscous fluid. Fd 6Rv s Fd is the frictional force – known as Stokes' drag – acting on the interface between the fluid and the particle (in N), •μ is the dynamic viscosity (N s/m2), •R is the radius of the spherical object (in m), and •vs is the particle's settling velocity (in m/s). Calculate the fall or settling velocity (Vt) for the given details through Stoke's Law formula. • Acceleration of Gravity (g) = 25 m/s2 Particle Diameter (d) = 15 m Density of Medium (ρm) = 5 kg/m4 Particle Density (ρp) = 10 kg/m3 Viscosity of Medium (μ) = 20 kg/m-s Vt = gd2 (ρp - ρm)/18μ Problem • Find the largest possible diameter for water drops falling in air with a velocity where Stoke’s law can be used In the calculation. Giver: density of air 1.2 kg/m3, μ – 1.8×10-5 kg/(m×s) Vt = gd2 (ρp - ρm)/18μ A terminal velocity is reached dx mg 1 f dt Where m – mass of particle - specific volume of colloidal particle - solvent density f – particle frictional factor Stokes Law • In the limit of – Slow particle motion – Dilute colloidal suspensions – Solvent is considered as a continuum of viscosity dx 2 R g 2 1 dt 9 2 dx 2 R g p f dt 9 2 The Frictional Factor • The frictional factor in a given medium is obtained from Stokes Law f 6R – solvent viscosity R- particle radius f, is a measure of the resistance to movement of a molecule; this resistance is a function of both the size and the shape of the molecule The Frictional Factor • D – diffusion coefficient • S – sedimentation coefficient Frictional Factors • Frictional factors depend on the particle shape • f increases as – Particle asymmetry increases – Degree of interaction with solvent increases • Define the frictional ratio, f/fo. – Ratio of the f value of the particle to that of an unsolvated sphere. Poiseuille's Law Calculation • In the case of smooth flow (laminar flow), the volume flowrate is given by the pressure difference divided by the viscous resistance. This resistance depends linearly upon the viscosity and the length, but the fourth power dependence upon the radius is dramatically different. Sedimentation of colloids buoyant mass F Vsed mg kT Fg 6 R ( Stokes) mg Vsed 6 R R 2 The bigger the particles the faster they sediment F F 15 Sedimentation • Under gravity – Balance method – cumulative mass of settling particles is obtained as a function of time – Practical lower limit is about 1 micron • Under centrifugal force – High Field – up to 4 x 105g is applied. – Displacement of boundary is monitored with time • Under low field – Measure concentration profile in the tube as a function of position. Erythrocyte sedimentation rate Erythrocyte sedimentation rate • Sedimentation rate or is the rate at which red blood cells sediment in a period of one hour. • To perform the test, anticoagulated blood is placed in an upright tube, known as a Westergren tube. The rate at which the red blood cells fall is measured and reported in mm/h. • The ESR is governed by the balance between prosedimentation factors, mainly fibrinogen, and those factors resisting sedimentation, namely the negative charge of the erythrocytes (zeta potential). • When an inflammatory process is present, the high proportion of fibrinogen in the blood causes red blood cells to stick to each other. The red cells form stacks called 'rouleaux,' which settle faster. Rouleaux formation can also occur in association with some lymphoproliferative disorders in which one or more immunoglobulin are secreted in high amounts. • The ESR is increased by any cause or focus of inflammation. The ESR is increased in pregnancy, inflammation, anemia or rheumatoid arthritis, and decreased in sickle cell anemia and congestive heart failure. The basal ESR is slightly higher in Diffusion • Diffusion is one of several transport phenomena that occur in nature. A distinguishing feature of diffusion is that it results in mixing or mass transport without requiring bulk motion. • There are two ways to introduce the notion of diffusion: either a phenomenological approach starting with Fick’s laws and their mathematical consequences, or a physical and atomistic one, by considering the random walk of the diffusing particles Fick’s Laws of Diffusion • The diffusion flux is proportional to the minus gradient of concentrations. It goes from regions of higher concentration to regions of lower concentration. c J D z Diffusion • From the atomistic point of view, diffusion is considered as a result of the random walk of the diffusing particles. In molecular diffusion, the moving molecules are self-propelled by thermal energy. Random walk of small particles in suspension in a fluid was discovered in 1827 by Robert Brown. Diffusion and Frictional Factors • The diffusion coefficient of a suspended particle is related to f via the Einstein Equation Df k BT For spherical particles k BT D 6R where D is the diffusion constant; μ is the mobility"; T is the absolute temperature kB is Boltzmann's constant; 1.3806503 × 10-23 m2 kg s-2K-1 Diffusion of ions through a membrane the flux is equal to mobility×concentration×force per gram ion. This is the so-called Teorell formula. The force under isothermal conditions consists of two parts: 1.Diffusion force caused by concentration gradient: 2.Electrostatic force caused by electric potential gradient: Here R is the gas constant, T is the absolute temperature, n is the concentration, the equilibrium concentration is marked by a superscript "eq", q is the charge and φ is the electric potential. Measurement of Diffusion Coefficients • Free boundary methods – a boundary between two solutions of different concentrations is formed in a cylindrical cell – Determine the evolution of the concentration distribution with time. Measurement of Diffusion Coefficients • Taylor Dispersion methods • NMR Techniques – Pulsed gradient spin echo experiments (PGSE) – Diffusion oriented spectroscopy (DOSY) http://www.youtube.com/watch?v=k5HMVIb4J7A&NR=1 Intensity of transmitted radiation related to solution turbidity () It e Io Nobel Prize for Chemistry for his work on the heterogeneous nature of colloidal solutions. Light Scattering and Colloidal Sizes • Size and shapes of colloidal systems can be obtained from scattering measurements • Advantages of Light Scattering – Absolute – No perturbations of system – Polydispersed systems – Fast Molar Masses from Scattering • Obtain the Rayleigh ratio at 90 16 R 90 3 1 Kc M R 90 2 no dn K 4 N A o dc 2 2 2 The Faraday-Tindall effect The distilled solution of absent, solve by water in different ratio Crab nebulosity Opal Tooth opalescence Light for the dental technician is essential, especially when it comes to aesthetics. In a healthy tooth light effects manifest themselves from inside. Separate layers of tissues react for the light at different angles. Interestingly, the structure of dentin and enamel differently behave to the light. Especially noticeable light blue opalescent glow enamel