* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
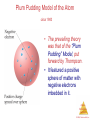
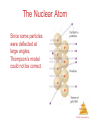
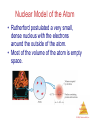
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 2 Atoms, Molecules, and Ions http://www.youtube.com/watch?v=vUzTQWn-wfE&feature=related Mrs. Deborah Amuso Camden High School Camden, NY 13440 Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Atomic Theory The earliest philosophers questioned: Can matter be subdivided into fundamental particles? Democritus (460-380 B.C.) – He coined the word ‘atomos’ – he said matter is made of tiny hard indivisible particles. Recall from Chapter 1: Atoms are the building blocks of matter. An atom is the smallest representative particle of an element. Atoms, Molecules, and Ions John Dalton John Dalton (1808) is famous for his Atomic Theory because his 4 key postulates helped to explain the known Laws of the time & actually predicted the Law of Multiple Proportions. Atoms, Molecules, and Ions Dalton's 4 Postulates 1. Elements are composed of atoms. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Dalton's 4 Postulates 2. All atoms of a given element are identical . Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Dalton's 4 Postulates 3. In chemical reactions atoms are not changed into different types of atoms. Atoms are neither created nor destroyed. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Dalton’s 4 Postulates 4. Compounds are formed when atoms of elements combine. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Law of Constant Composition Joseph Proust (1754–1826) • This is also known as the Law of Definite Proportions. • It states that the elemental composition of a pure substance never varies. • In a given compound, the relative numbers and kinds of atoms are always the same, regardless of its source. • Vitamin C is always C3H4O3 Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Law of Conservation of Mass • Matter cannot be created or destroyed during ordinary chemical reactions, the atoms simply rearrange to form new substances. • Total mass before Rx = Total mass after Rx • 5 grams A + 7 grams B = 12 grams AB Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Law of Multiple Proportions • When elements A and B are able to form more than one compound: The masses of B than can combine with a given mass of A are in small whole number ratios. • For example: CO (carbon monoxide) & CO2 (carbon dioxide) • Here’s the proof using sulfur-fluorine compounds as an example: Compound 1: 23.2 g S & 55.0 g F Compound 2: 16.6 g S & 9.8 g F Compound 3: 19.3 g S & 68.6 g F Atoms, Molecules, and Ions To Prove the Law • Step 1: Find the F/S ratio Compound 1: 55.0/23.2 = 2.37 Compound 2: 9.8/16.6 = 0.59 Compound 3: 68.6/19.3 = 3.55 • Step 2: Find simplest ratio Compound 1: 2.37/0.59 = 4 Compound 2: 0.59/0.59 = 1 Compound 3: 3.55/0.59 = 6 • Surprised? The compounds are SF4, SF, and SF6 Atoms, Molecules, and Ions J. J. Thompson J. J. Thompson (1897) He used Cathode Ray Tubes to measure the charge/mass ratio for an electron. He is credited with discovery of the electron. This lead to the Plum-Pudding Model of the atom. http://www.youtube.com/watch?v=YG-Wz-arcaY&feature=related Atoms, Molecules, and Ions The Electron • Streams of negatively charged particles were found to emanate from cathode tubes. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Plum Pudding Model of the Atom circa 1900 • The prevailing theory was that of the “Plum Pudding” Model, put forward by Thompson. • It featured a positive sphere of matter with negative electrons imbedded in it. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Robert Millikan Robert Millikan (1909) – He determined the charge on an electron during his famous Oil Drop Experiment. http://www.youtube.com/watch?v=XMfYHag7Liw Atoms, Molecules, and Ions Millikan Oil Drop Experiment Charge on the electron is 1.602 x 10-19 Coulombs Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Radioactivity • Radioactivity is the spontaneous emission of radiation by an atom. • It was first observed by Henri Becquerel. • Marie and Pierre Curie also studied it. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Radioactivity Three types of radiation were discovered by Ernest Rutherford: particles (positively charged) = Alpha particles (negatively charged) = Beta rays (not a particle – no charge) = Gamma http://www.youtube.com/watch?v=vuGvQjCOdr0&feature=related Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Ernest Rutherford Ernest Rutherford (1911) – His Gold Foil Alpha Scattering Experiment led to the discovery of protons and the nucleus – He showed that atoms have a positive center and are mostly empty space. http://www.youtube.com/watch?v=5pZj0u_XMbc Atoms, Molecules, and Ions Discovery of the Nucleus Ernest Rutherford shot particles at a thin sheet of gold foil and observed the pattern of scatter of the particles. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. The Nuclear Atom Since some particles were deflected at large angles, Thompson’s model could not be correct. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Nuclear Model of the Atom • Rutherford postulated a very small, dense nucleus with the electrons around the outside of the atom. • Most of the volume of the atom is empty space. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Bohr Model of the Atom Niels Bohr (1913) – His work studying light given off by excited atoms led to the proposal that electrons move around the nucleus in specific electron shells. http://www.youtube.com/watch?v=wCCz20JOXXk Atoms, Molecules, and Ions James Chadwick James Chadwick (1932) – He discovered neutrons Atoms, Molecules, and Ions Most Modern Model of the Atom The Modern Model – also called Electron Cloud Model or Orbital Model or Wave Mechanical Model – This model says it is impossible to know exactly where an electron is at any given time. Electrons are in regions of space called orbitals. You will learn more about this model & the scientists that contributed to the development of this model in Chapter 6. Atoms, Molecules, and Ions The Periodic Table 1. Arranges the elements by increasing ATOMIC NUMBER (not mass). 2. GROUPS (vertical columns) contain atoms having the same # of valence electrons & similar chemical properties. 3. PERIODS (horizontal rows) contain atoms having the same # of occupied electron shells. Their properties vary from metal to metalloid to nonmetal. Atoms, Molecules, and Ions Periodicity When one looks at the chemical properties of elements, one notices a repeating pattern of reactivities. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Group Names to Know Group 1 = Alkali Metals Group 2 = Alkaline Earth Metals Groups 3 to 12 = Transition Metals Group 16 = Chalcogens Group 17 = Halogens Group 18 = Noble Gases Atoms, Molecules, and Ions Classes of Elements 1. METALS – have luster, malleable, ductile, conductors of heat & electricity. 2. METALLOIDS – have properties of both metals & nonmetals. 3. NONMETALS – if solid, they are brittle & poor conductors. 4. NOBLE GASES – inert (don’t react) Atoms, gases Molecules, and Ions Location • METALS – left of staircase (except hydrogen which is a nonmetal) • NONMETALS – right of staircase and hydrogen • METALLOIDS – on staircase (B, Si, Ge, As, Sb, Te) • NOBLE GASES – in Group 18 (often considered nonmetals) Atoms, Molecules, and Ions Periodic Table Nonmetals are on the right side of the periodic table (with the exception of H). Notice that some textbooks consider the noble gases to also be nonmetals. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Periodic Table Metalloids border the stair-step line (with the exception of Al, Po, and At). Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Periodic Table Metals are on the left side of the chart. (except H) Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Subatomic Particles 1. Proton – Positive Charge, Mass = about 1 amu 2. Neutron – Neutral, Mass = about 1 amu 3. Electron – Negative Charge, Mass = 1/1836 amu Atoms, Molecules, and Ions Atoms: Bohr Model Summary 1. While most of the volume of the atom is empty space, atoms do have a central dense positive nucleus (containing positive protons & neutral neutrons). 2. Negative electrons circle the nucleus in specific energy levels. 3. Each atom is NEUTRAL because its # of protons equals its # of electrons. 4. All atoms of a given element have the same ATOMIC NUMBER (same # of protons). Atoms, 5. The MASS NUMBER is the sum of the Molecules, # of protons + # of neutrons in an atom. and Ions Symbols of Elements Elements are symbolized by one or two letters. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Atomic Number All atoms of the same element have the same number of protons: The atomic number (Z) Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Atomic Mass The mass of an atom in atomic mass units (amu) is the total number of protons and neutrons in the atom. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Atomic Mass Atomic and molecular masses can be measured with great accuracy with a mass spectrometer. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Isotopes 1. ISOTOPES are atoms of the same element with different masses due to differences in # of neutrons. 2. Notation: 3 1H 3 = mass number 1 = atomic number 3. Another way: H-3 Atoms, Molecules, and Ions Average Mass • Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. • Average mass is calculated from the isotopes of an element weighted by their relative abundances. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Atomic Mass 1. The mass of an atom is measured in a unit called the amu. 2. One ATOMIC MASS UNIT (amu) = 1/12 the mass of a carbon-12 atom or 1.66 x 10-24g. 3. The ATOMIC MASS or AVERAGE ATOMIC MASS of an element is calculated from the relative abundances and atomic masses of that element’s isotopes. Atoms, Molecules, and Ions Calculating Avg. Atomic Mass (also called atomic weight – AW) There are 3 steps: 1) For each isotope, multiply its atomic mass times it percent abundance. 2) Add each of these values together. 3) Divide by 100 to obtain the avg atomic mass if you didn’t use the % sign. AW = [(atomic mass)(%)] + [(atomic mass)(%)] Then, ask yourself if your answer for the average makes sense? Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Example Rubidium has 2 naturally occurring isotopes. Rb-85 (atomic mass = 84.9118 amu, abundance 72.15%) and Rb-87 (atomic mass = 86.9092 amu, abundance 27.85%) 1. Calculate its atomic weight using: AW = [(atomic mass)(%)] + [(atomic mass)(%)] 2. Set-up: AW = [(84.9118)(72.15%)] + [(86.9092)(27.85%) 3. Answer: AW = (61.26) + (24.20) = 85.46 amu* *If you got 8546 amu for the answer, you need to divide by 100 Atoms, Molecules, and Ions Calculating P, E, N for Neutral Atoms # Protons = Atomic Number (42) # Electrons = Atomic Number (42) Mass number = Atomic Mass rounded-off to nearest whole number (96) # Neutrons = Mass Number – Atomic Number (96 – 42 = 54) Atoms, Molecules, and Ions Chemical Formulas 1. The subscript to the right of the symbol of an element tells the number of atoms of that element in one molecule of the compound. 2. There are 2 basic types of compounds: Molecular or Ionic. 3. There are 3 types of chemical formulas: molecular, empirical, & structural. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Types of Compounds: Molecular or Ionic Molecular compounds are composed of molecules and almost always contain only nonmetals. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Types of Compounds: Molecular or Ionic Diatomic Molecules: These seven elements occur naturally as molecules containing two atoms. Remember: HOFBrINCl While these are elements and not compounds, they should be mentioned at this time because they are also examples of molecules. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Types of Compounds: Molecular or Ionic Ionic compounds (such as NaCl) are generally formed between metals and nonmetals and/or polyatomic ions. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Types of Formulas: Empirical, Molecular, or Structural • Empirical formulas give the lowest whole-number ratio of atoms of each element in a compound. • Molecular formulas give the exact number of atoms of each element in one molecule of a compound. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Examples • Molecular Formulas for Molecular Compounds CO2 , NH3 CH4 , HCl • Empirical Formula for an Ionic Compound NaCl Atoms, Molecules, and Ions Types of Formulas: Empirical, Molecular, or Structural • Structural formulas show the order in which atoms are bonded. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Ions • Ions are atoms that have lost or gained their electrons and as a result are no longer neutral in charge. • The resulting ion has an octet (8 valence electrons). • Metals lose electrons & form positive ions. • Nonmetals gain electrons & form negative ions. Atoms, Molecules, and Ions Ions – Memorize these! Cations are positive and are formed by elements on the left side of the periodic chart. Anions are negative and are formed by elements on the right side of the periodic chart. These ions are formed from a single atom and are called Monoatomic ions. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Ions with Multiple Charges • Usually transition metals • Two Examples: Memorize these two! Cuprous (Cu 1+) vs Cupric (Cu 2+) Ferrous (Fe 2+) vs Ferric (Fe 3+) • Pattern: Lois Lane is superman’s girlfriend. The lower charge is the –ous ion. Get it? Low-ous (Lois) Atoms, Molecules, and Ions Polyatomic Ions 1. Polyatomic Ion - A group of covalently bonded atoms that have lost or gained electron(s) and as a result have an electronic charge. 2. Example: SO4 2- = Sulfate Ion Atoms, Molecules, and Ions Common Anions - Memorize Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Common Cations - Memorize Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Finding # P, E, N for ions Recall - For Neutral Atoms: # p = atomic number # e = atomic number # n = mass number – atomic number Ions are atoms that have lost or gained electrons. While the # p and # n in the atom and its corresponding ion are the same, Atoms, the # e differs. Molecules, and Ions © 2009, Prentice-Hall, Inc. Finding # P, E, N for ions Positive cations are formed from atoms that have LOST electrons. 23 11Na atom vs 23 1+ ion Na 11 Atom: 11 Protons, 12 Neutrons, 11 Electrons The +1 charge on the ion means it has one less electron. Ion: 11 Proton, 12 Neutron, 10 Electrons Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Finding # P, E, N for ions Negative anions are formed from atoms that have GAINED electrons. 35 17Cl atom vs 35 1- ion Cl 17 Atom: 17 Protons, 18 Neutrons, 17 Electrons The -1 charge on the ion means it has 1 extra electron. Ion: 17 Proton, 18 Neutron, 18 Electrons Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Review: Formulas for Compounds • Molecular formulas tell the exact # and kind of atom in one molecule of the compound. Molecular compounds contain only nonmetallic elements. Examples: H2O or C6H12O6 • Empirical formulas tell the simplest whole number ratio between the atoms in the compound. Ionic compounds contain Atoms, metals and nonmetals and/or polyatomicMolecules, and Ions ions. Examples: NaCl or NH4Br Writing Formulas • Because compounds are electrically neutral, one can determine the formula of a compound by criss-crossing ion charges. – The charge on the cation becomes the subscript on the anion. – The charge on the anion becomes the subscript on the cation. – If these subscripts are not in the lowest wholenumber ratio, divide them by the greatest common Atoms, factor. Molecules, and Ions © 2009, Prentice-Hall, Inc. Steps to Writing Formulas 1. 2. 3. 4. List symbol and charge of cation. List symbol and charge of anion. If either is polyatomic put ( ) around it. If there is a Roman numeral in the name, it tells the charge on the cation. 5. Criss-cross charges. 6. Simplify. Example #1: Barium Phosphate Ba2+ (PO4)3- Ba3(PO4)2 Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Writing Formulas Example #2: Calcium Sulfide Ca2+ S2- Ca2S2 simplify CaS Example #3: Magnesium Hydroxide Mg2+ (OH)1- Mg(OH)2 Use must keep ( ) Example #4: Iron (III) Oxide – also named ferric oxide Fe3+ O2- Fe2O3 Example #5: Copper (II) Sulfate – also named cupric sulfate Cu2+ (SO4 )2- Cu2(SO4 )2 simplify CuSO4 Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Steps to Nomenclature (Naming) 1. Write the name of the cation. 2. If the anion is an element, change its ending to -ide; if the anion is a polyatomic ion, simply write the name of the polyatomic ion. 3. If the cation can have more than one possible charge, write the charge as a Roman numeral in parentheses. Example #1: (NH4)2CO3 Ammonium Carbonate Example #2: AgBr Silver Bromide Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Nomenclature (Naming) Example #3: NaC2H3O2 Sodium Acetate Example #4: FeCl2 Iron (II) Chloride or Ferrous Chloride Example #5: Sr(NO3)2 Strontium Nitrate Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Oxyanion Patterns 1. Oxyanions = Negative ions that contain oxygen 2. Knowing this naming pattern can help you memorize the ion names more easily. 2. Look at the pattern using chlorine w/ oxygen. Chloride ion = Cl 1Hypochlorite ion = ClO 1Chlorite ion = ClO2 1Chlorate ion = ClO3 1Atoms, Molecules, Perchlorate ion = ClO4 1and Ions Some Common Oxyanions Nitrite = NO2 1- Nitrate = NO3 1- one more oxygen Sulfite = SO3 2- Sulfate = SO4 2- Note: The –ite ion doesn’t always have 2 oxygen atoms. However the -ate ion always has 1 more oxygen atom than the –ite ion! Atoms, Molecules, and Ions You try: 1. If bromate ion = BrO3 1-, then bromite = ? ans. BrO2 12. If carbonate = CO3 2- and hydrogen = H 1+, then what is the hydrogen carbonate ion? ans. HCO3 1Another name for hydrogen carbonate is bicarbonate. Atoms, Molecules, and Ions Acid Nomenclature • If the anion in the acid ends in -ide, change the ending to -ic acid and add the prefix hydro- . – HCl: hydrochloric acid – HBr: hydrobromic acid – HI: hydroiodic acid Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Acid Nomenclature • If the anion in the acid ends in -ite, change the ending to -ous acid. – HClO: hypochlorous acid – HClO2: chlorous acid Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Acid Nomenclature • If the anion in the acid ends in -ate, change the ending to -ic acid. – HClO3: chloric acid – HClO4: perchloric acid Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Steps: Nomenclature of Binary Molecules 1. Binary Molecules contain just 2 different nonmetals. 2. The less electronegative atom is usually listed first. 3. A prefix is used to denote the number of atoms of each element in the compound (mono- is not used on the first element listed, however). 4. The ending on the more electronegative element is changed to –ide. CO2 carbon dioxide CCl4 carbon tetrachloride Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Nomenclature of Binary Molecules Note: If the prefix ends with a or o and the name of the element begins with Memorize these prefixes! a vowel, the two successive vowels are often elided into one. N2O5 dinitrogen pentoxide SO3 sulfur trioxide* *Don’t confuse this with the polyatomic ion SO3 2as SO3 is a neutral molecule. Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc. Nomenclature • Also be able to name or given formulas for these compounds: Water = H2O Methane = CH4 Ammonia = NH3 Hydrogen Peroxide = H2O2 Atoms, Molecules, and Ions © 2009, Prentice-Hall, Inc.