* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Branchial Cleft Cysts
Survey
Document related concepts
Transcript
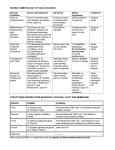
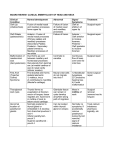
Branchial Cleft Cysts July 10,1997 Thanh Nguyen, M.D. Terminology The word branchial comes from the Greek, "bragchia," meaning gills. A cyst refers to a mucosa or epithelium lined structure with no external or visceral openings. A sinus refers to a tract with or without a cyst that communicates to either the gut or skin. A fistula is a tract connecting the gut to the skin. Embryology Structures between the developing head and the heart (i.e., the face, neck, oropharynx, and the larynx) develop from the branchial apparatus. There are six branchial arches; the last two are rudimentary. Each arch has a bar of mesoderm. Caudal to each of the four arches is an internal pouch lined with entoderm. Externally is branchial cleft, lined with ectoderm. Between each bar, a branchial plate, composed of entoderm and ectoderm, separates the branchial cleft from the branchial pouch. In the fish, these branchial plates rupture to form gill slits. In the human, they do not rupture and are soon invaded by the mesoderm of the neighboring arches. It is more accurate to omit the word "cleft" when describing branchial anomalies, as this implies that all of the malformations are derived solely from a branchial cleft with no contribution from the corresponding arch or pouch. Branchial clefts 2, 3, and 4 fuse into one structure referred to as the cervical sinus (of His) created by downward growth of the overlapping second arch's ventral pole. The latter becomes the platysma muscle. This stage is important in understanding how many of these anomalies come to lie deep to the platysma. It is generally believed that the branchial anomalies are derived from remnants of this sinus. During the growth of a massive first arch, there is a slow movement away from the pericardium. The interval between is filled by the gradual enlargement of the epicardial ridge structure. The muscle cells from this ridge form the infrahyoid muscles and the sternomastoid muscle supplied by the hypoglossal nerve. The first branchial arch on each side divides into maxillary and mandibular processes. The mesenchyma of the mandibular arch transforms into Meckel's cartilage. The second branchial arch gives rise to Reichert's cartilage. One would expect a first cleft defect to lie between the main derivatives of Meckel's and Reichert's cartilages. Also, there should be close association with the external auditory canal, since the external canal is a derivative of the first cleft. The parotid gland is also closely related to any first branchial anomaly. The relationship of a first branchial anomaly to the parotid gland will be variable because the parotid gland has a somewhat later embryologic development. Likewise, the relationship of a first branchial anomaly to the facial nerve will also be variable because the VIIth cranial nerve and its muscles migrate upward at the 6th to the 8th week. Fistulas of the second arch would have an external opening at the anterior border of the sternomastoid because this muscle mass arises from the epicardial ridge. This is also the reason for the fistula's position superficial to the hypoglossal nerve because this nerve supplies muscles from the epicardial ridge. The tract then passes deep to the platysma since this muscle is derived from the second branchial bar. The tract ascends along the carotid sheath passing deep to the external carotid system but superficial to the internal carotid artery. The internal carotid artery derives from the third branchial bar primitive artery. The tract continues to ascend superficially to the glossopharyngeal nerve and the stylopharyngeus muscle, also third branchial derivatives. The tract may perforate the tonsillar fossa, which is the area of the second branchial cleft. A cyst may lie anywhere along this tract. A fistula formed from the third branchial arch has its external opening in the same area as the second branchial fistula. The tract passes deep to the platysma, ascending along the common carotid sheath, but this time passing behind the internal carotid artery. The tract crosses the hypoglossal nerve but will not ascend above the glossopharyngeal nerve or the stylopharyngeus muscle, which are third branchial bar derivatives. The tract is superficial to the superior laryngeal nerve that supplies fourth branchial bar derivatives. The internal opening is in the pyriform sinus, the area formed from the third branchial cleft. Fourth pouch sinuses also arise from the pyriform sinus but in contrast they course inferior to the superior laryngeal nerve. Complete fourth branchial apparatus anomalies have never been conclusively demonstrated. Theoretically, because of their site of origin, fistulas or sinus tracts originating in this branchial region would loop around the right subclavian artery on the right or the aortic arch on the left, and course superiorly to the upper esophagus. Histology Branchial cysts are usually lined by squamous epithelium -- about 90% of the cases. Eight percent of them are composed of ciliated columnar epithelium; and two percent show both types of epithelium. They are characterized by considerable quantities of subepithelial lymphoid tissue, generally in a follicular pattern with germinal centers or, less frequently, in a diffuse bandlike pattern. Occasionally present are salivary tissue, sebaceous glands, and cholesterol clefts with a foreign body reaction. Usually the lumen is filled with viscid yellow fluid characteristically containing large amounts of glittering cholesterol crystals. The branchial sinuses are more likely to show a pseudostratified columnar lining. Lymphoid tissue is generally present, although it usually lacks the well structured follicular arrangement seen in their cystic counterparts. These and other differences lead some authors to doubt a common etiology for branchial sinuses and cysts. Clinical Presentation Neel and Pemberton recorded the symptoms and frequency in 319 cases of lateral cervical cysts or fistulas. A typical lateral cervical fistula is present at birth. Cysts tended to present later than sinuses or fistulae. This is not surprising, for without a sinus or a fistulous tract there is less chance of infection or early recognition. Most cysts occur in the region of the lower pole of the parotid gland, as have other reported cystic anomalies. A branchial cyst most commonly presents in the second through fourth decades of life. Males and females are equally affected and there is occasionally a hereditary tendency. Twenty to 40 percent of the patients relate its discovery to an attack of pharyngitis, ear infection, or dental infection, and many report temporary enlargement with or without tenderness during periods of upper respiratory tract infection. Inflamed cysts may progress to abscess formation with the possibility that rupture or incision and drainage will lead to either permanent sinus formation or to recurrent cyst formation and infection. Larger cysts may displace the sternomastoid muscle posterolaterally and the carotid and internal jugular vein medially. With any further increase in the size of the cyst, the deep cervical fascia prevents its expansion anteriorly between the sternomastoid muscle and the strap muscles of the larynx. As a result, the cyst takes the path of least resistance and extends posterior and medial to the sternomastoid. Olsen et al showed that first cleft cysts occurred twice as often as sinuses or fistula. Proctor and Proctor have shown that second branchial cleft cysts occur three times more often than second branchial sinuses or fistulas. Five cases of bilateral branchial cysts have been reported. Interestingly, these may be a familial connection. Ninety-five percent of branchial anomalies are second branchial anomalies. Upon reviewing the world literature, Kaneko et al and Richardson et al report approximately 50 cases of first branchial cleft cysts. One of these cases was reported by Dr. Shirley in the Baylor ENT residency program. Third and fourth branchial arch anomalies are rare (Doi et al, 1988) and only 31 reports of such branchial arch anomalies are recorded, all of which have been sinuses (Takimoto et al, 1990). A complete fistula, although theoretically feasible, has never been demonstrated. The sinuses usually present with recurrent episodes of neck abscess or acute suppurative thyroiditis, a perithyroid abscess or a retropharyngeal abscess. These abnormalities occur on the left hand side in 93% of the 28 reported cases (Godin et al, 1990), and typically present either in the neonatal period or in older children (3 to 11 years). This leftsideness is probably due to the asymmetry of transformation of the fourth branchial arch to form the aorta and innominate arteries. Classification of First Branchial Anomalies Arnot proposed the first classification for anomalies of the first branchial cleft. He designated as a Type 1 defect any cyst or sinus in the parotid gland that is lined by squamous epithelium and which presents in early or middle adult life. Type 2 defects develop during childhood in the anterior triangle of the neck, with a communicating tract to the external auditory canal. However, the most widely accepted classification theory is that of Work. Type I anomalies are believed to be solely of ectodermal origin and duplications of the membranous EAC. These lesions are usually located in the preauricular area, and they tend to be parallel to the EAC. A fistula or sinus tract may course to end deep in the EAC or middle ear. Type II first branchial anomalies are also considered to be duplications of the EAC, but these lesions are derived from both ectoderm and mesoderm. These anomalies are usually located posterior and/or inferior to the angle of the mandible. A fistula or sinus tract may course toward the EAC and open more laterally near the bone and cartilage junction. Both types of first branchial cysts, sinuses, and fistulas are intimately involved with the parotid gland and facial nerve. Type I lesions are extremely rare. Type II variety are more numerous. Finn et al state that neither type of branchial cleft cyst is associated with pretragal cysts or sinuses. These arise from failure of fusion of the auricular hillocks around the dorsum of the first branchial arch. They are always lateral to the facial nerve and, although they may arborize, they are not related to the external auditory canal. Arnot, Work, Belensky and Medina classifed first cleft anomalies. Arnot based his classification primarily on anatomical location, whereas Work classified defects according to histology, implying that embryological differences give rise to the two types. These authors arrived at similar classifications, and correlated the anatomical site and the histopathology of the defect. As Belensky and Medina illustrated, many cases do not fit into either Arnot's or Work's classifications, because of the lack of correlation between site of occurrence and histopathology of the anomaly. Olsen et al maintain that such a classification does not aid in the diagnosis or surgical management of such defects. They separate first cleft anomalies into cysts, sinuses, or fistulae without regard to anatomical site or histological subtypes. Differential Diagnosis Differential diagnosis includes: branchiogenic carcinoma, tuberculous adenitis, lipoma, metastatic malignant neoplasms (SCCA from a primary site in the aerodigestive tract), cystic hygroma (lymphangioma), carotid body tumors, lymphomas, hemangiomas, thyroid cysts, ectopic thyroid, cervical thymic cysts, thyroglossal duct cyst, parotid cystic tumors. The wall of tuberculous fisulas is very irregular compared with the smooth wall of a branchial fistula. Diagnostic Studies In cases of sinus or fistula, especially in third and fourth branchial anomalies, sinogram or barium contrast study can delineate the course of the anomaly. Barium swallow should be performed after the resolution of acute inflammation in order to decrease the chance of false-negative results. For branchial cysts, diagnostic studies have been less helpful. Ultrasonography cannot differentiate between a branchial cyst and cystic metastases. CT is likewise unhelpful. Fine needle aspiration is of limited value. Granstrom and Edstrom (1989) studied 42 patients with lateral cervical cystic masses, nine of which proved to be malignant - only three of these FNA's were positive. They found positive results in masses present for the longest period of time. Endoscopy and biopsy of suspicious areas only can produce a false sense of security. Because the vast majority of cystic metastases are attributable to a malignancy of Waldeyer's ring (Batsakis, 1981), endoscopy and ipsilateral tonsillectomy and blind biopsies of Waldeyer's ring are recommended in the patients older than 40 years. Branchio-Oto-Renal Syndrome is an autosomal, dominant disorder. The branchial manifestations are usually inconsequential; however, the hearing impairment and renal malformations can be significant. The concept of "branchiogenic carcinoma" was first introduced by Volkman in 1882 to explain 3 cases of deeply infiltrating cervical carcinoma without identifiable primary malignancies. In 1950, Martin et al published a landmark paper on branchiogenic carcinoma, which set the tone of most subsequent reports in the literature. They reviewed 250 cases of carcinoma of branchial cysts reported in the literature, and identified only three cases among them that could presumably be considered legitimate. Strict criteria for the establishment of a diagnosis of branchial cleft carcinoma have been forwarded: 1. Location of the tumor in the anatomic region of the branchial cleft cyst or sinus. 2. Histologic appearance of the tumor consistent with its origin from branchial vestiges; i.e., squamous cell carcinoma. 3. Presence of the carcinoma within the lining of identifiable epithelial cyst. 4. Identification of transition from the normal squamous epithelium of the cyst to carcinoma. 5. Absence of any identifiable primary malignant tumor after exhaustive evaluation of the patient. Treatment Surgery is indicated for branchial anomalies because there is a lack of spontaneous regression, a high rate of recurrent infection, the possibility of other diagnoses, and rare malignant degeneration. There is little disagreement that the treatment of choice is complete surgical excision of the cyst or sinus tract. If any debate exists, it would be about when to operate. If branchial fistulas, sinuses, or cysts are noted in the neonatal period, surgery can be postponed until three to six months of age. This allows the child to grow and hopefully precedes a first upper respiratory infection which may lead to infection. If the abnormality is noted after six months of age, surgery can be done as soon as possible. Most surgeons believe that the presence of a branchial cyst or sinus is the only necessary indication for surgery. The reason for this early excision is the high incidence of secondary infections of these lesions probably at least 25% before definitive surgery. Infection contributes to morbidity both in its own right and more importantly because it doubles the recurrence rate after surgery by adding to the difficulty of the excision. Other primary treatments, including radiation therapy, injection of sclerosing agents, and repeated incision and drainage or aspiration, are not only noncurative but also markedly increase the recurrence rate after surgery. In the Mayo Clinic series of 283 branchial remnant resections, the recurrence rate was 21% when there was a history of surgery and 14% when there was a history of infection, compared with a recurrence of 3% for cases with no history of surgery or infection. Recurrences are about twice as likely in surgery of branchial sinuses as in surgery of branchial cysts. First branchial remnants are often closely associated with the facial nerve and external auditory canal. Identification and dissection of the facial nerve is a necessary step. The procedure is identical to that used at parotidectomy, but the dissection rarely needs to extend more than 1-2 cm peripheral to the division of the facial nerve. Some authors recommend superficial parotidectomy, but this has not always been necessary. Because the ear canal cartilaginous duplication anomaly frequently communicates with the cartilaginous meatus, radical operation often results in a defect in the cartilaginous meatus. However, it has been a general experience that such defects heal without problems and without subsequent stenosis after packing the meatus for 3-4 weeks. The standard surgery for second arch anomaly is usually by a stepladder incision originally described by Bailey in 1933. Visualization of the tract at operation may be aided by injecting into the fistula paraffin, methylene-blue dye or quick-hardening polymers. The dissection may be facilitated by prior catheterization of the fistula. Alternatively, fistulas can be excised through the oral route. Stripping of the fistula, first described by Heanley in 1976, requires a correct diagnosis and a complete fistula with both internal and external openings (a complete fistula with external and internal openings is rare). It involves insertion of the stripper from the cervical end into the pharynx, freeing of the cervical end, fixation of the cervical end to the stripper, avulsion by gentle steady traction on the oral end, and closure of the cervical wound. Invagination of the fistula was described by Von Hacker in 1897. The fistula was mobilized from below as far possible towards the pharynx and then excised. A probe was passed through the residual portion of the fistula into the mouth. The fistula was then inverted into the mouth and ligated. In 1963, Cox inserted the stripper along the outside of the tract then avulsed it from below. The per-oral excision of a branchial cyst has been described by Takimoto et al, 1989, and Dilkes et al, 1990. The operation is only possible when the cyst lies in a medial position, close to the pharyngeal wall. The presence of a branchial cyst in such a medial position supports the congenital theory for its etiology. This is because lymph nodes are not known to exist in the parapharyngeal tissue around the tonsil, and the position of the cyst in this case represents the medial limit of the second branchial cleft. Functional neck dissection is suitable for treatment of recurrent cervical branchial defects. For resection of third or fourth branchial remnants, the pyriform sinus opening can be approached by cutting a window in the lateral ala of the thyroid cartilage. Because the dissection extends into the tracheoesophageal groove, the recurrent and superior laryngeal nerves, as well as the parathyroid glands, are at risk of injury during exploration. Additionally, consideration must be made for performing a hemithyroidectomy if there is a history of suppurative thyroiditis. History Although almost all surgeons agree that congenital lateral cervical sinuses and fistulas result from anomalies of the branchial apparatus, there are many theories for the etiology of lateral cervical cysts. They can be grouped into two categories: the congenital and the cervical lymph nodes cystic transformation theories. The most widely held belief is that cervical cysts are derived from the branchial apparatus (Hosemann and Wigand, 1988). Cysts presenting in the lateral aspect of the neck were first described by Hunczovsky (1785). At the beginning of the 19th century, Rathke (1828) described the development of the pharyngeal arches in the human fetus. Shortly after, Ascherson (1832) described 11 cases of branchial fistulae. Ascherson equated the development of lateral cervical cysts with that of branchial fistulae due to their location. Everything appeared to fit nicely into this neat categorization until 1912 when Wenglowski published the results of his dissection of cadavers and human embryos, which led to doubts about the branchiogenic origin of many lateral cervical cysts. He showed that pharyngeal cleft tissue was not represented in any adult tissue inferior to the hyoid bone. Thus any cyst lying below this level could not be derived from a pharyngeal cleft. Wenglowski went on to describe the development of the thymus from the third pharyngeal pouch via the thymopharyngeal duct. He suggested that incomplete obliteration of the thymopharyngeal duct resulted in a lateral cervical cyst. An alternative theory suggests that lateral cervical cysts represent cystic lymph nodes. However, it has been pointed out that cystic transformation of lymph nodes is not known to occur in other anatomical sites. Luck (1861) noted that the external appearance of a lateral cervical cyst showed a great similarity to a hypertrophied lymphatic gland. Luschka (1848) described a lymph node between the external and internal carotid arteries. King (1949) studied 76 cervical cysts and concluded that they had no direct relationship with any structure in the embryo. Instead he emphasized the close relationship between these cysts and lymphoid tissue. Bhaskar and Bernier (1959) reviewed the histology of 468 cysts: 452 had a wall composed of lymphoid tissue. They suggest that the cystic alteration of cervical lymph nodes is stimulated by trapped epithelium. This hypothesis has become known as the "Inclusion Theory." They originally suggested three possible sources for these epithelial inclusions: brachial cleft, pharyngeal pouch and parotid gland. Another explanation is that, as a result of tonsillitis and pharyngitis, squamous epithelium derived from the pharynx spreads via the lymphatic system to regional lymph nodes. Subsequent growth of this epithelium and cystic degeneration of the node forms the cyst (Wilde et al, 1987). At present, to clarify the origin of these cysts, immunohistochemistry is being used to study, at the molecular level, the nature of the epithelial cells and their relationship to other types of epithelium elsewhere in the body. Case Presentation A 2-year and 9-months-old male, former 36-week premature infant that at birth was noted to have midfacial and mandibular hypoplasia consistent with the diagnosis of Treacher-Collins Syndrome. The family history was negative for relatives with craniofacial or auricular malformations. His past medical history was notable for uncomplicated neonatal jaundice. Medical work-up for congenital cardiac and renal anomalies was negative. On exam, the patient is noted to have malar hypoplasia, absent zygomatic arches, hypoplasia of the mandible with Class III occlusion, and bilateral Grade III microtia with aural atresia. The mastoids, however, are well developed bilaterally. A tracheotomy is present in the neck. The remainder of the physical exam was unremarkable. Upper airway obstruction secondary to severe mandibular hypoplasia necessitated elective tracheotomy in the first week of life. A gastrostomy tube was placed secondary to poor oral intake. At two months of age, he underwent audiometric evaluation consisting of air and bone conduction multichannel ABR. The patient was fitted with a bone-conducting type hearing aid at age four months. Subsequently, the patient underwent repair of his palatal defect at age two, and most recently underwent bilateral Ilizarov mandibular distraction procedures. After more than two years with his bone-conducting aids, he has acquired comprehensible speech and a vocabulary comparable to that of children with normal hearing. Bibliography Andrews JC, Anzai Y, Mankovich NJ, Favilli M, Lufkin RB, Jabour B. Three-dimensional CT scan reconstruction for the assessment of congenital aural atresia. Am J Otol 1992;13:236-240. Chang SO, Min YG, Kim CS, Koh TY. Surgical management of congenital aural atresia. Laryngoscope 1994;104:606-611. Cole, RR. The "buttock sign" in congenital aural atresia. Otolaryngol Head Neck Surg 1993;109:140-141. Cole RR, Jahrsdoerfer RA. The risk of cholesteatoma in congenital aural stenosis. Laryngoscope 1990;100:576-578. Cole RR, Jahrsdoerfer RA. Congenital aural atresia. Clin Plast Surg 1990;17:367-371. Cressman WR, Pensak ML. Surgical aspects of congenital aural atresia. Otolaryngol Clin North Am 1994;27:621-633. Fuente del Campo A, Elizondo MM, Arnaud E. Treacher Collins syndrome (mandibulofacial dysostosis). Clin Plast Surg 1994;21:613-623. Glasscock MR, Jackson CG, Nissen AJ, Schwaber MK. Management of congenital ear malformations. Ann Otol Rhinol Laryngol 1983;92:504-509. Granstrom G, Bergstrom K, Tjellstrom A. The bone-anchored hearing aid and bone-anchored epithesis for congenital ear malformations. Otolaryngol Head Neck Surg 1993;109:46-53. Hall JW, Morgan SH, Aguilar EA, Mackey J, Jahrsdoerfer RA. Neuro-otologic applications of simultaneous multi-channel auditory evoked response recordings. Laryngoscope 1994;94:883889. Hayes D. Hearing loss in infants with craniofacial anomalies. Otolaryngol Head Neck Surg 1994; 110:39-45. Jahrsdoefer RA, Hall JW. Congenital malformations of the ear. Am J Otol 1986;7:267-269. Jahrsdoerfer RA, Garcia ET, Yeakley JW, Jacobson JT. Surface contour three-dimensional imaging in congenital aural atresia. Arch Otolaryngol Head Neck Surg 1993;119:95-99. Jahrsdoerfer RA, Worth JW, Aguilar EA, Cole RR, Gray LC. Grading system for the selection of patients with congenital aural atresia. Am J Otol 1992;13:6-12. Jahrsdoerfer RA. Congenital atresia of the ear. Laryngoscope 1978;88:1-49. Jones RL. Smith's Recognizable Patterns of Human Malformation, 4th ed. Philadelphia: Saunders, 1988:709. Lambert PR. Congenital Aural Atresia. In: Bailey BJ., et al. Head and Neck Surgery Otolaryngology. Philadelphia, . Lippincott, 1993:1579-1591. Mattox DE. Fisch U. Surgical correction of congenital aural atresia of the ear. Otolaryngol Head Neck Surg 1986;94:574-577. Molony TB, De la Cruz A. Surgical approaches to congenital atresia of the external auditory canal. Otolaryngol Head Neck Surg 1990;103:991-1001. Pattee GL. An operation to improve hearing in cases of congenital atresia of the external auditory meatus. Arch Otolaryngol 1947;46:568-580. Patten BM. Human Embryology. New York: McGraw-Hill, 1968:335-344. Schuknecht HF. Congenital aural atresia. Laryngoscope 1989;99:908-917. Shambaugh GE. Surgery of the Ear. Philadelphia: Saunders, 1959:493-523. Shih L, Crabtree JA. Long-term surgical results for congenital aural atresia. Laryngoscope 1993; 103:1097-1102. Swartz JD, Harnsberger HR. Imaging of the Temporal Bone. New York: Thieme Medical Publishers, 1992:20. Portmann M, Guillen G, Richards AE. The Ear and Temporal Bone. New York: Masson, 1979:382-384.