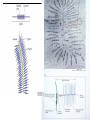
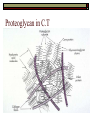
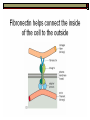
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download BCH 450 CAT 1 lectures
Biochemistry wikipedia , lookup
Cell theory wikipedia , lookup
Cell culture wikipedia , lookup
Developmental biology wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Mineralized tissues wikipedia , lookup
Wound healing wikipedia , lookup
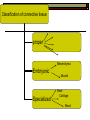
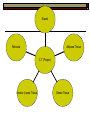
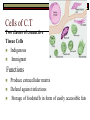
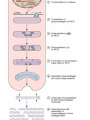
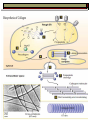
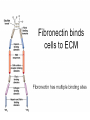
BCH 450 Specialized Tissues Dr. Samina Hyder Haq Dept of Biochemistry King Saud University Recommended Books - Mammalian Biochemistry of Specialized Tissues by Smith & Hill Principles of biochemistry. Lehninger. , Nelson & Cox. Harper's Biochemistry. Robert K. Murray , Daryl K. Granner , Peter A. Mayes , Victor W. Rodwell. Syllabus 1. Topics to be covered Lectures 1.What isConnective tissues 2 2.Collagen detailed structure , composition ,biosynthesis, function. at cellular level. 3.Epithelial tissues& muscles tissues 4.Nervous tissues 5.Kidney 6. Liver 5 5 6 3 3 What is Connective tissues Connective tissue is a form of fibrous tissue. It is one of the four types of tissue in traditional classifications (the others being epithelial, muscle, and nervous tissue). These tissues form a framework, or matrix, for the body, and are composed of two major structural protein molecules, collagen and elastin Collagen is the main protein of connective tissue in animals and the most abundant protein in mammals, making up about 25% of the total protein content. What is the function of connective tissue 1.Supporting and protecting: The minerals and fibers produced by connective tissue cells establish a bony structural framework for the body, protect delicate organ, and surrounding and interconnect other tissue. 2.Transporting materials: Fluid connective tissue provides an efficient means of moving dissolved materials from one region of the body to another. 3. Storing energy reserves. Fats are stored in connective tissues called adipose cells until needed. Adipose cells- stores fat in connective tissue until needed. 4. Defending the body. Specialized connective tissue cells respond to invasions by microorganism through cell-to-cell interactions and the production of antibodies. Connective tissues- are the most diverse tissues of the body, comprised of bone, blood, and fat are some of the examples. Components of Connective tissue All connective tissues have three basic components: 1.Specialized cells 2. Protein fibers 3. A ground substance- is a fluid that varies in consistency. Classification of Connective tissue Connective tissue can be classified into three categories: proper, embryonic, specialized Classification of connective tissue proper Mesenchyma Embryonic Mucoid Specialized Bone Cartilage Blood Elastic Reticular Adipose Tissue C.T (Proper) Areolar (Loose Tissue) Dense Tissue Connective Tissue proper Connective tissue proper includes the following five types: loose connective, dense connective, elastic, reticular, and adipose.They are called "proper" because they are the types usually meant when using the phrase "connective tissue Areolar or Loose Tissue Areolar tissue exhibits interlacing,loosely organized fibers,abundant blood vessels, and significant empty space. Its fiber run in random directions and are mostly collagenous, but elastic and reticular fibers are also present. Areolar tissue is highly variable in appearance. In many serous membranes, it appears as a loose arrangement of collagenous and elastic fibers, scattered cells of various types; abundant ground substance; numerous blood vessels. In the skin and mucous membranes, it is more compact and sometimes difficult to distinguish from dense irregular connective tissue. Dense Fibrous C.T Dense connective tissue, also called dense fibrous tissue, has collagen fibers as its main matrix element. It is mainly composed of collagen type I. Crowded between the collagen fibers are rows of fibroblasts, fiber-forming cells, that manufacture the fibers. Dense connective tissue forms strong, rope-like structures such as tendons and ligaments. Tendons attach skeletal muscles to bones; ligaments connect bones to bones at joints. Ligaments are more stretchy and contain more elastic fibers than tendons. Dense connective tissue also make up the lower layers of the skin (dermis), where it is arranged in sheets Elastic Fibre Elastic fibers (or yellow fibers) are bundles of proteins(elastin) found in extracellular matrix of connective tissue and produced by fibroblasts and smooth muscle cells in arteries. These fibers can stretch up to 1.5 times their length, and snap back to their original length when relaxed. Elastic fibers include elastin, and oxytalan. Reticular connective tissue is a type of connective tissue proper. It has a network of reticular fibers, made of type III collagen that form a soft skeleton to support the lymphoid organs (lymph nodes, bone marrow, and spleen.) Reticular fibers are synthesized by special fibroblasts called reticular cells. The fibers are thin branching structures, Adipose Tissue contains adipocytes, used for cushioning, thermal insulation, lubrication (primarily in the pericardium) and energy storage. Embryonic Connective tissue Mesenchyme, or mesenchymal connective tissue, is an example of reticular connective tissue, a type of loose connective tissue, which is derived from all three germ layers and located within the embryo. 2. Mucous connective tissue (or mucous tissue) is a type of connective tissue found during fetal development 1. Specialized C.T(Blood. Bone & Cartilage) Blood functions in transport. Its extracellular matrix is blood plasma, which transports dissolved nutrients, hormones, and carbon dioxide in the form of bicarbonate. The main cellular component is red blood cells. The following two can be classified as "supportive connective tissue": 2. Bone (osseous tissue) makes up virtually the entire skeleton in adult vertebrates. 3. Cartilage makes up virtually the entire skeleton in chondrichthyes. In most other vertebrates, it is found primarily in joints, where it provides cushioning. The extracellular matrix of cartilage is composed primarily of collagen 1. Basic Elements of Connective tissue 1. 2. Cells Extra cellular Matrix consists of GAGS Ground Substance PG Adhesive glycoprotein Collagen Tissue Fluids Fibres Elastic Reticular Cells of C.T Two classes of connective Tissue Cells Indigenous Immigrant Functions Produce extracellular matrix Defend against infections Storage of foodstuffs in form of easily accessible fats Indigenous cells of connective tissue Undifferentiated mesenchymal cells, proliferate and differentiate into fibroblasts , fat cells, chondrocytes. Fibroblasts: Spindle shaped cells with big ER and Golgi. Synthesize most of ECM molecules (especially collagen) Proliferate and migrate in response to tissue injury. Mast cells:Immune cells Mediate immediate hypersensitivity reactions Secrete histamine and proteases Cause plasma extravasation (oedema), itching Indigenous cells of connective tissue Adipocytes (white fat cells) Mostly just under the skin Store lipids in the form of single droplet Effector cells for insulin Take up glucose and synthesize triglycerides for storage in the lipid droplet Also endocrine organ – secrete leptin, a hormone responsible for appetite regulation Different from brown fat cells that oxidize lipids to produce heat Immigrant cells of connective tissue Blood cells Granulocytes 1. Monocytes and macrophages 2. Neutrophils 3. Lymphocytes 4. Basophils 5. Eosinophils All derive from a stem cell in bone marrow Origin of C.T Cells Undifferentiated mesenchymal cells Collagen Fibres Collagen is the most abundant protein in the human body .It consist of about 25% of the total protein in our body. It is one of the long, fibrous structural proteins whose functions are quite different from other proteins; There is a great heterogeneity in the structure and fibril structure of collagen which varies from great tensile strength of Bone to the delicate structure of cornea and retina of the eye. Types of Collagen The collagen super family of proteins includes more than twenty eight collagen types, as well as additional proteins that have collagen-like domains. 1. Fibril forming Collagen Network Forming Collagen Fibril associated collagen 2. 3. Taken from Lippincot’s biochemistry Major Types of Collagen Type Composition Distribution I [α1(I)]2α2 Skin, tendon,bone,cornea II [α1(II)]3 III [α1(III)]3 Cartilage, vitreous body, intervertebral disc Fetal skin, cardiovascular system IV [α1(IV)3 Basement membrane The Family of Genetically Distinct Collagen Types Cont;(kielty &Grant) Schematic representation of the domain structures and relative dimensions of the different molecules of the classified collagen family (types I– XIX; groups 1–3). Triplehelical domains are represented by shaded bars and non-helical sequences as solid lines. Lengths of triplehelical domains (nm) are indicated by arrows With complements from Kielty &Grant (2002) Structure of collagen (Amino acid sequence Collagen is rich in proline and glycine, both of which are important in the formation of the triple-stranded helix. Proline facilitates the formation of the helical conformation of each α chain because its ring structure causes “kinks” in the peptide chain. Glycine, the smallest amino acid, is found in every third position of the polypeptide chain. It fits into the restricted spaces where the three chains of the helix come together. The glycine residues are part of a repeating sequence, -Gly–X–Y–, where X is frequently proline and Y is often hydroxyproline (but can be hydroxylysine,). Most of the α chain can be regarded as a polytripeptide whose sequence can be represented as (–Gly–Pro–Hyp–) Proline & hydroxyproline Collagen Biosynthesis Transcription(mRNA) Translation (protein ) Nucleus RER (cytosol Post translational modification 1.Hydroxylation of proline or lysine 2. Glycosylation (addition of glucose and galactose to hydroxylysine) Lateral assembly and secretion from golgi complex as procollagen Cleavage of N and C terminus propeptide by Collagenase In extra cellular matrix Assembly of tropocollagen as collagen fiber in ECM Collagen biosynthesis (courtesy of Lippencot biochemistry) Biosynthesis of Collagen Fibril assembly More than 25 different alpha chains create >20 collagens Type I usually associated - Dermatan Sulfate – dermis, bone, tendon, fibrocartilage Type II usually associated - Chondroitin Sulfate – hyaline & elastic cartilage Type III usually associated - Heparan Sulfate – Reticular fibers - very fine - Type III collagens endoneurium, liver, spleen, kidney Type IV usually associated - Heparan Sulfate Tropocollagen as the basic structural unit of Collagen Tropocollagen has a mass of about 285 kdal and consist of three polypeptide chains. Tropocollagen - 280 nm long - head & tail region • 30% glycine, 30% proline & hydroxyproline • re-aggregate - native collagen (64nm) Non Covalent hydrogen bonding between the three α chain is via hydroxyproline. tropocollagen polarized in fiber, 1/4 staggered array – period accounted for by gaps fall in dark bands . Staggered array of tropocollagen Molecules Collagen fibres exhibit crosss striations every 640A. The length of the tropocollagen molecule s 2800A. There is a gap of 400oA between the end of one tropocollagen and the start of another. This gap play an important role in mineralization process. Tropocollagen Assembly Fibril arrangement of different types of Collagen I,II,III.V, XI VII,X IV VI VII FACIT Collagens I, II, III, V, and XI form fibrils as a consequence of the staggered alignment of molecules. Type IV collagen forms a flexible open network through N- and Cterminal and helical interactions, and this is the basic structural framework of basement membranes. Thin microfibrils of type VI collagen represent the end-product of a complex pathway of assembly, whereby monomers associate to form dimers, which, in turn, come together to form tetramers able to associate end to end. Type VII collagen anchoring fibrils are created as a result of monomers overlapping at their C-termini to form centrosymmetric dimers capable of aligning in limited parallel arrays. Triple-helical domains are shown as shaded bars, and non collagenous domains as lines. The closely related types VIII and X collagens form open hexagonal arrays. The FACIT collagens (fibril-associated collagens with interrupted triple helices) (types IX, XII, XIV) do not form defined assemblies on their own but instead are thought to associate with the surface of fibrils. Type IX collagen molecules are cross-linked to the outermost fibrillar collagen molecules of type II collagen fibrils. Cu+2 containing E Cross linking of collagen Lysyl oxidase is an extracellular copper enzyme that catalyzes formation of aldehydes from lysine residues in collagen and elastin precursors.These aldehydes are highly reactive, and undergo spontaneous chemical reactions with other lysyl oxidase-derived aldehyde residues, or with unmodified lysine residues. This results in crosslinking collagen and elastin, which is essential for stabilization of collagen fibrils and for the integrity and elasticity of mature elastin. Complex cross-links are formed in collagen called Pyridinoline which is derived from three lysine residues Stability of the collagen helix™ The temperature at which half of the helical structure is lost is called the melting temperature™. The Tm of tropocollagen is a criterion of the stability of its helical structure. Tm depends on the body temperature of the source species. collagens from icefish has the lowest Tm while warm blooded animals have the highest Tm. This difference in thermal stability is correlated with the contents of imino acid (proline and hydroxy proline) in the collagen. The higher the imino acid content , the more stable the helix. Tm of (pro-pro-Gly) is 24C while poly (Pro-HypGly) is 580C indicating hydroxylation stabilizes triple helix. The experiments using αα\-bipyridyl an iron chelator which inhibit hydroxylation shows that without hydroxylation triple helix formation does not occur. What is scurvy Scurvy is the connective tissue disorder in which body failed to synthesise normal collagen resulting in formation of weak and brittle bones, weak blood vessels with a frequent haemorrhages and bleeding. Scurvy ( deficiency of vitamin C Biochemical basis of Scurvy: Vitamin C maintains prolyl hydroxylase and lysine hydrxylase in an active form keeping the iron atom in its reduced ferrous state Lysine hydroxylase Lysine hydroxylysine Vit.C + Fe+2 Proline hydroxylase Proline Hydroxyproline Vit.C + Fe+2 Other heritable disorder of connective Tissue. Marfan syndrome - a genetic disease causing abnormal fibrillin. Ehlers-Danlos syndrome - causes progressive deterioration of collagens, with different EDS types affecting different sites in the body, such as joints, heart valves, organ walls, arterial walls Osteogenesis imperfecta (brittle bone disease) caused by insufficient production of good quality collagen to produce healthy, strong bones. Stickler syndrome- affects collagen, and may result in a distinctive facial appearance, eye abnormalities, hearing loss, and joint problems Ehlers-Danlos syndrome This disorder is a heterogeneous group of generalized connective tissue disorders that result from inheritable defects in the metabolism of fibrillar collagen molecules. EDS can result from a deficiency of collagen-processing enzymes (for example, lysyl hydroxylase deficiency or procollagen peptidase deficiency), or from mutations in the amino acid sequences of collagen types I, III, or V. The most clinically important mutations are found in the gene for type III collagen. Collagen containing mutant chains is not secreted, and is either degraded or accumulated to high levels in intracellular compartments. Because collagen type III is an important component of the arteries, potentially lethal vascular problems occur. Extra cellular Matrix GAGS( Glycosoaminoglycan), are un branched polysaccharides with repeating disaccharide units. They resist compression and fill space. Three Common GAGS What are GAGS Glycosaminoglycans (GAGs) Negatively charged Extended conformation They are hydrophilic attract cations and water to form hydrated gel. Difficult to compress Packing materials, ground substances present in Cartilages, cornea What are Proteoglycan? Proteoglycan = core protein + GAG subunit GAG sub unit. is unbranched repeat disaccharides. Chondroitin Sulfate , Dermatan Sulfate, Heparan Sulfate, Keratan Sulfate • GAG sub unit. covalently linked to core-protein like bristles on brush Proteoglycans Proteoglycan in C.T Adhesive glycoprotein 1. 2. Cell adhesive glycoprotein have binding domains for cell surface integrins & for components of the E.C.M. Most important are Fibronectin Laminin Laminin Laminin-(basal lamina)- cruciate shaped with 3 peptide chains - binding sites for integrin, heparan sulfate, type IV collagen & entactin