* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Power Point Notes
Survey
Document related concepts
Transcript
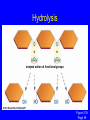
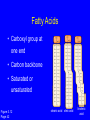
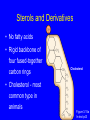
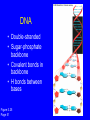
Molecules of Life Chapter 3 3.1 Organic Compounds Hydrogen and other elements covalently bonded to carbon Carbohydrates Lipids Proteins Nucleic Acids Carbon’s Bonding Behavior • Outer shell of carbon has 4 electrons; can hold 8 • Each carbon atom can form covalent bonds with up to 4 atoms Methane: Simplest Organic Compound H H C H Ball-and-stick model H Structural formula Space-filling model Figure 3.2 Page 36 Bonding Arrangements • Carbon atoms can form chains or rings • Other atoms project from the carbon backbone Glucose (ball-and-stick model) In-text figure Page 36 Hemoglobin Molecular Models Ball-and-stick model Space-filling model Ribbon model Figure 3.3 Page 37 3.2 Functional Groups • Atoms or clusters of atoms that are covalently bonded to carbon backbone • Give organic compounds their different properties Examples of Functional Groups Methyl group - CH3 Hydroxyl group - OH Amino group - NH3+ Carboxyl group - COOH Phosphate group - PO3- Sulfhydryl group - SH 3.3 Types of Reactions Functional group transfer Electron transfer Rearrangement Condensation Cleavage Condensation Reactions • Form polymers from subunits • Enzymes remove -OH from one molecule, H from another, form bond between two molecules • Discarded atoms can join to form water Condensation enzyme action at functional groups enzyme action at functional groups Figure 3.7a Page 39 Hydrolysis • A type of cleavage reaction • Breaks polymers into smaller units • Enzymes split molecules into two or more parts • An -OH group and an H atom derived from water are attached at exposed sites Hydrolysis enzyme action at functional groups Figure 3.7b Page 39 3.4 Carbohydrates Monosaccharides (simple sugars) Oligosaccharides (short-chain carbohydrates) Polysaccharides (complex carbohydrates) Monosaccharides • Simplest carbohydrates • Most are sweet tasting, water soluble • Most have 5- or 6carbon backbone Structure of glucose Disaccharides • Type of oligosaccharide • Two monosaccharides covalently bonded • Formed by condensation reaction glucose fructose + H2O sucrose Figure 3.8b Page 40 Polysaccharides • Straight or branched chains of many sugar monomers • Most common are composed entirely of glucose Figure 3.9 Page 40 Starch chain Cellulose & Starch • Differences in bonding patterns between monomers yield different properties cellulose Figure 3.10 Page 41 amylose (a starch) Glycogen • Sugar storage form in animals • Large stores in muscle and liver cells Figure 3.10 Page 41 Chitin • Polysaccharide • Nitrogen-containing groups attached to glucose monomers • Structural material for hard parts of invertebrates, cell walls of many fungi 3.5 Lipids • Most include fatty acids – Fats – Phospholipids – Waxes • Sterols and their derivatives have no fatty acids • Tend to be insoluble in water Fatty Acids • Carboxyl group at one end • Carbon backbone • Saturated or unsaturated Figure 3.12 Page 42 stearic acid oleic acid linolenic acid Fats • Fatty acid(s) attached to glycerol • Triglycerides are most common Figure 3.13 Page 42 Phospholipids • Main component of cell membranes • Hydrophilic head • Hydrophobic tails Fig. 3.14a,b Page 43 Sterols and Derivatives • No fatty acids • Rigid backbone of four fused-together carbon rings Cholesterol • Cholesterol - most common type in animals Figure 3.15a In-text p43 Waxes • Long-chain fatty acids linked to long-chain alcohols or carbon rings • Firm consistency, repel water • Important in water-proofing 3.6 Amino Acid Structure Carboxyl group Amino group Figure 3.16 Page 44 R group tryptophan (trp) Figure 3.17 Page 44 Protein Synthesis • Peptide bond – Condensation reaction links amino group of one amino acid with carboxyl group of next Water forms as a by-product Fig. 3.18a Page 45 Primary Structure • Sequence of amino acids • Unique for each protein • Two linked amino acids = dipeptide • Three or more = polypeptide • Backbone of polypeptide has N atoms: -N-C-C-N-C-C-N-C-C-N- 3.7 Second and Third Levels • Hydrogen bonding produces helix or sheet • Domain formation Tertiary structure Secondary structure Figure 3.19a Page 46 Fourth Level Structure Some proteins are made up of more than one polypeptide chain Figure 3.20 Page 47 HLA-A2 quaternary structure Hemoglobin alpha chain beta chain beta chain alpha chain 3.8 One Wrong Amino Acid • Single amino acid change in beta chain can cause sickle-cell anemia HbS valine histidine leucine proline threonine valine glutamate Fig. 3.21c,d Page 48 Sickle Cell Anemia • Caused by two mutated copies (HbS) of Hb gene • Low oxygen causes red blood cells to clump • Clumping prevents normal blood flow • Over time, may damage tissues and organs throughout the body 3.9 Nucleotide Structure • Sugar • At least one ATP phosphate group • Nitrogen- containing base Figure 3.23a Page 50 Nucleotide Functions • Energy carriers • Coenzymes • Chemical messengers • Building blocks for nucleic acids DNA • Double-stranded • Sugar-phosphate backbone • Covalent bonds in backbone • H bonds between bases Figure 3.25 Page 51 RNA • Usually single strands • Four types of nucleotides • Unlike DNA, contains the base uracil in place of thymine • Three types are key players in protein synthesis