* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chirality in Pharmaceutical Synthesis
Survey
Document related concepts
Pharmaceutical marketing wikipedia , lookup
DNA-encoded chemical library wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Psychopharmacology wikipedia , lookup
Development of analogs of thalidomide wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Environmental persistent pharmaceutical pollutant wikipedia , lookup
Transcript
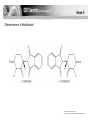
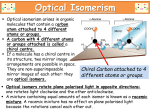
Chirality in Pharmaceutical Synthesis • Explain that the synthesis of pharmaceuticals often requires the production of a single optical isomer. • Explain that synthetic molecules often contain a mixture of optical isomers, whereas natural molecules often have only one optical isomer. • Explain that the synthesis of a pharmaceutical that is a single optical isomer increases costs, reduces side effects and improves pharmacological activity. • Describe strategies for the synthesis of a pharmaceutical with a single optical isomer. Week 9 Pharmacological activity depends on whether or not a drug can interact with a receptor site in a biological system. © Pearson Education Ltd 2009 This document may have been altered from the original Optical Isomerism in Nature • Many of the natural biochemical reactions which take place in living systems require molecules of a specific shape for reactions to occur. E.g. neurotransmitters interact with sites on nerve cells which have to fit exactly both the shape and intermolecular forces of the molecules. • This is true for all “lock and key” interactions. • Dopa is a neurotransmitter which exhibits optical isomerism – only the L form is active in the brain. • The structure of L-Dopa follows. Draw the displayed formula for this molecule. Identify the chiral centre. Draw the 2 stereoisomers on either side of a mirror plane. Optical Isomerism in Nature • L-Dopa is used as a treatment for Parkinson’s Disease. • It must be free of D-Dopa which has unpleasant side effects. • Undesirable side effects were a particular problem with the drug Thalidomide, prescribed as a sedative and anti-emetic (to prevent morning sickness) for pregnant women in the 1960s. • One stereoisomer was effective but the other proved to be teratogenetic. Week 9 Stereoisomers of thalidomide © Pearson Education Ltd 2009 This document may have been altered from the original Modern Pharmaceuticals • Computers are now used to examine the relationship between a molecule and a receptor site. • Molecular modelling has greatly speeded up the process of designing new medicines. • Now only molecules that show potential after computer tests are made and tested in the real world. • If a single stereoisomer can be made risks from undesirable side effects are reduced. • The risk of litigation is reduced. Modern Pharmaceuticals • Drug dosages are reduced (by half) which does reduce some packaging costs. • However the synthesis of single isomer drugs is more expensive due to the need to separate the optical isomers. • Most synthetic routes involve reactions of functional groups where incoming reagents can attack from the ‘back’ or the ‘front’ of the molecule. This causes the production of the racemic mixtures. Modern Pharmaceuticals • Complicated separation techniques are required to separate the enantiomers because they usually have very similar physical properties. • Methods used include enzyme reactions, electrophoresis and chromatography. • These are expensive and time consuming. Modern Chiral Synthesis • Single isomer preparation methods used include: • Using bacteria or enzymes as catalysts since they are all stereospecific. • Use of natural α-amino acids and sugars as starting materials – so-called chiral pool synthesis. • Use of transition metal catalysts (chiral catalysts) particularly using supercritical carbon dioxide as solvent. • Use of strained cyclic molecules (like cyclopropane) which can only react on one ‘side’ of the molecule because the other is blocked. • Using reagents fixed onto polymer supports with reactants flowing over them.