* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download MALAYSIA: PRODUCT REGISTRATION AND REGULATION
Tablet (pharmacy) wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacognosy wikipedia , lookup
Compounding wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
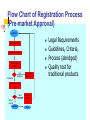
CONTROL OF DIETARY SUPPLEMENTS IN MALAYSIA TAN LIE SIE DRUG EVALUATION AND SAFETY DIVISION NATIONAL PHARMACEUTICAL CONTROL BUREAU MINISTRY OF HEALTH MALAYSIA Seminar on dietary supplements - 15 January 2004 CONTENTS Food / Drug Interface Regulatory Process in Malaysia Concerns Conclusion FOOD/DRUG INTERFACE What is this interface? - interaction of 2 regulatory regimes; - no internationally standardised approach; - complex, with public health and safety implications Guide to Classification of Food-Drug Interface Products (Guide to determining if a product is to be regulated by the NPCB/FQC) Product Ingredients Pure Form of active ingredient (singly or in combination) Vitamin mineral amino acid Fatty acid Fibre LyophilizedBacteria Enzyme Natural product that are not traditionally used as food and of medicinal value Alfalfa tablets Spirulina Royal Jelly Noni Juice Pegaga tablet Herbal product NPCB-National Pharmaceutical Control Bureau Less than 80% food base or more than 20% active ingredients of natural products. But if the latter possess high potencies, the product shall be reviewed by the Committee, even if they contain less than 20% of these ingredients. Food base 80% or more Food base FQC – Food Quality Control Division • If a product is more than 80% food based but contains pure forms of active ingredient (e.g.: vitamins & minerals) that exceed the amounts permitted in Food Regulations 1985, the company shall be advised to reduce the amounts of these active ingredients and be regulated by FQC. • Intended use and claim should not be used as sole criteria for classification but can be used as a guide • Instruction for use and pharmaceutical dosage form like tablet, capsule, should not be used as criteria for classification but can be used as a guide. FOOD/DRUG INTERFACE If a product contains less than 80% of foodbased ingredients and more than 20% of the active ingredients, such product shall be regulated by NPCB. Not withstanding this general rule, for specific ingredients which possess high potencies, even if they contain less than 20% of the active ingredients, they shall be reviewed by the committee and may be regulated by NPCB if it is found necessary FOOD/DRUG INTERFACE If a product is more than 80% food based but contains pure forms of active ingredient (e.g.: vitamins & minerals) that exceed the amounts permitted in Food Regulations 1985, the company shall be advised to reduce the amounts of these active ingredients and be regulated by BKMM. FOOD/DRUG INTERFACE Following criteria should not be used as sole criteria for classification but can be used as a guide Intended use and claim Instruction for use and pharmaceutical dosage forms like tablet, capsule, etc DRUG CONTROL AUTHORITY NATIONAL PHARMACEUTICAL CONTROL BUREAU (NPCB) (as Secretariat / Executive Arm) DRUG CONTROL AUTHORITY (DCA) Register all drugs (prescription, over-thecounter and herbal medicines) and cosmetic products License manufacturers, importers and wholesalers of registered products Monitor the quality and safety of marketed products through Post-Registration Market Surveillance & Adverse Drug Reactions Reporting The Control of Drugs and Cosmetics Regulations 1984 Regulation 7(1)(a) requires ALL products to be registered with the DCA prior to being imported, manufactured, sold or supplied, unless the product is exempted under specific provisions of these Regulations. The Control of Drugs and Cosmetics Regulations 1984 A “product”as defined in the Regulations, means “a drug in a pharmaceutical dosage form, or a cosmetic, having a singular identity, composition, characteristics and origin”. The Control of Drugs and Cosmetics Regulations 1984 A drug is used on humans (and animals) to prevent, cure, treat, or reduce illness , to diagnose disease, for contraception, to induce anaesthesia (sedate), to change or to control physiological function, to control body weight, general maintenance or promotion of health or well being DIETARY SUPPLEMENTS Products intended to supplement the diet, taken by mouth in forms such as pills, capsules, tablets, liquids or powders and not represented as conventional food. May include ingredients such as Vitamins, Minerals, Amino Acids, Natural Substances of plant/animal origin, Enzymes Substances with nutritional / physiological function Why register dietary supplements? Need to protect consumer interests * Record of products registered and their respective responsible market authoriztion holders and manufacturers * Monitoring and enforcement * Regulate claims * Safety aspects REGISTRATION CRITERIA SAFETY Products will not be registered if there are public health concerns based on safety considerations (ingredients used, combinations) Upper daily limits set for some vitamins and minerals Warnings/precautions may be required in product labelling REGISTRATION CRITERIA QUALITY Have to comply with current Good Manufacturing Practices (cGMP) requirements - infrastructure /facilities - personnel - processes and controls Should conform to set standards of quality - raw materials - finished product - stability testing REGISTRATION CRITERIA CLAIMS ( EFFICACY ) Supplements may not bear disease claims (ie capable of curing, treating or preventing disease); either explicit or implied Allowed to be indicated as “Dietary / Food/ Nutritional Supplement” Function claims which describe the physiological role of the nutrient in normal functioning of the body may be permitted REGISTRATION PROCESS On-line registration process (single stage) Implementation of the on-line registration system : 1 July 2003 - for pharmaceuticals (generics), and the “OTC” products 1 January 2004 - for traditional products 1 Mac 2004 - targeted date for the NCE and Biotech products Flow Chart of Registration Process (Pre-market Approval) Applicant Obtain smart card Input data & submit BPFK evaluate application Satisfactory Not satisfactory Request for additional info Prepare evaluation report JKPP Report complete Report incomplete Secretariat Request for additional info DCA Legal Requirements Guidelines, Criteria, Process (abridged) Quality test for traditional products REGISTRATION PROCESS Unique registration no MAL20001198X Registration for a maximum period of 5 years Updating of product information Re-register before expiry of term to be maintained on product register CONCERNS Consumer perception that a product in the form of a “medicine” is perceived to be a drug for treatment Responsible information provision. There is potential high risk to consumers as a result of insufficient or incorrect information, or fraudulent products. - Although a product may not be toxic or dangerous, consumers may compromise their health by not seeking proper medical attention. - Claims about supplements should not divert attention away from eating a healthy diet CONCLUSION There is tremendous interest in and demand for health supplements and a need for regulators to accommodate both the industry and consumers in this area. CONCLUSION However the growing market for supplements in a less restrictive regulatory environment creates the potential for supplements to be prone to quality-control problems CONCLUSION Authority to regulate and approve product registration and licensing through pre-market assessment helps ensure that consumers have access to safe, high quality, properly labeled products. http: // www.bpfk.gov.my