* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharm Science,technology and public health
Survey
Document related concepts
Transcript
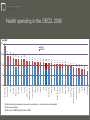
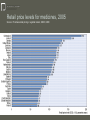
What is in the Price of Medicines? The costs and benefits to civil society of the research based pharmaceutical industry Brussels 26th November 2008 David Taylor Professor of Pharmaceutical and Public Health Policy, The School of Pharmacy, University of London This presentation This contribution discusses issues relating to Belgian and EU wide public policy on pharmaceutical pricing and spending. It attempts to build an understanding of the cost of medicines today within a broad social and economic development framework Professor Sir Michael Rawlins and Mr Oscar Wilde The public has multiple, sometimes conflicting, interests in medicines and health care. They range from ensuring optimally affordable access to existing treatments to investing in therapeutic and scientific progress for future generations “A fool knows the price of everything and the value of nothing." – Medicines can serve not only as treatments for individuals but as stepping stones to overall public health improvement. They are atypical high technology products. Once developed and introduced to the medical market they can normally be copied at relatively low cost. In economic terms, their supply typically involves high ‘sunk’ costs and low marginal costs of production – The inherent complexity of many policy issues in the pharmaceutical sector can be a barrier to balanced public debate. But a failure today to value pharmaceuticals appropriately could impose very significant costs on twenty first century Europe George Orwell and streptomycin in 1947/48 Agenda Medicines in health improvement Pharmaceutical prices and spending in Belgium Issues relating to medicines pricing at the European Union and global levels Some suggested topics for further discussion Stages of health development –Demographic transition (Warren Thompson, 1929) –Epidemiological transition (Abdel Omran, 1972) –Care transition, and the processes of social adjustment to increased wealth and longevity King James 1 granting the British Apothecaries their first Royal Charter in 1617 Dimensions of later stage ‘care transition’ include….. – Increasingly assertive consumerism in health care – greater demands for both personal autonomy and medicines safety and effectiveness, even in extreme circumstances – Decreased tolerance of gross health inequalities, and higher expectations of universal care access – A shift of aspects of care quality protection from professional to regulatory agency and managerial control For sources see, for instance, the work of Inglehart, Giddens, Sennet, Flynn and Salter In the nineteenth and early twentieth centuries European health improvement was primarily driven by better nutrition and factors such as enhanced sanitation, housing and child care Since the 1940s we have experienced health challenges associated with ‘wealthy’ lifestyles, counterbalanced by the development and use of presently available medicines The next few decades should see more effective support for ‘healthy’ lifestyles (with a trend towards the elimination of tobacco use, increased exercise rates and better diets), coupled with the earlier and better targeted use of future medicines to fit the requirements of specific phenotypes Medicines pricing in Belgium –Medicine prices are set by the Minister of Economic Affairs, and reimbursement levels by the Minister of Social Affairs. In most instances these are the same –When a generic variant of an original medicine becomes available because of patent expiry, the reimbursement base rate of all forms of the original is reduced by 30% –There is a further 14% price cut when a molecule has been reimbursed for more than 12 years and additional 2.3% price cut when it has been reimbursed for more than 15 years Belgian market outcomes – Overall, medicine prices in Belgium are – whichever way they are calculated – close to the European average. But within that overall picture prices for patented medicines are relatively low in international terms, while those for off-patent medicines are relatively high – There is significant use of off-patent branded products, supplied at the ‘reference reimbursement’ (generic price) level – The country spends a little over 1 per cent of its GDP on pharmaceuticals at manufacturers’ prices, which is in line with OECD average data – In per capita terms, Belgium is one of the world’s major beneficiaries from pharmaceutical exporting and enjoys significant pharmaceutical sector investment and employment 0 8.9 8.8 8.7 8.4 8.4 8.3 8.2 8.2 OECD Australia (3) Norway Spain United Kingdom Hungary Finland Japan (4) 6.4 6.2 Korea Poland 5.7 6.6 Mexico Turkey (4) 6.8 Czech Republic 7.1 9.0 Italy Slovak Republic (4) 9.1 Iceland 7.3 9.1 Greece Luxembourg 9.2 Sweden 7.5 (1) Public and private components are current expenditure,i.e. investments are not separated. (2) Current expenditure. (3) Data refer to 2005/06. (4) Data refer to 2005. Source: OECD Health Data 2008 , June 2008. Ireland 9.3 New Zealand 10.0 Canada 9.3 10.1 Austria Netherlands (2) 10.2 Portugal 9.5 10.4 Belgium (1) Denmark (1) 10.6 Germany 11.1 4 France 8 11.3 12 Switzerland United States 15.3 Health spending in the OECD, 2006 Chart 2: Health Expenditure as a Share of GDP, 2006 % of GDP 16 Public Private Pharmaceutical spending in total health spending Source: Pharmaceutical pricing in a global market, OECD, 2008 Pharmaceutical spending in Belgium Proportion of pharmaceutical specialities expenditures in % of total public healthcare expenditures 1990-2007 Mio EUR En % 20.000 19 18.000 16.000 17 14.000 12.000 15 10.000 8.000 13 6.000 4.000 11 2.000 0 9 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 Public healthcare expenditure (INAMI) Pharmaceutical specialities expenditures Proportion of pharmaceutical specialities expenditures in total public healthcare expenditures (in %) Effective proportion of the pharmaceutical industry in the total public healthcare expenditures (in %) 2005 2006 2007 Pharmaceutical retail price components Source: Pharmaceutical pricing in a global market, OECD, 2008 Retail price levels for medicines, 2005 Source: Pharmaceutical pricing in a global market, OECD, 2008 Pharmaceutical sector trade balances, 2003 According to (2002) Eurobarometer data some 65% of people in Belgium believe their health system runs well, or is in need of only minor changes. This compares with an EU average of 44%, around 30% in the UK and Italy and 14% in Portugal. However, more recent EHCI data suggest that Belgian consumer satisfaction levels may be declining, in part due to delays in access to new medicines Issues relating to medicines pricing internationally The research based pharmaceutical industry’s cost structure – an illustrative outline Total production costs Promotion and information Research and development Other costs Profit 30 per cent of gross income? 25 per cent? 15 - 20 per cent? 10 - 15 per cent? 10 - 15 per cent? Cost structures vary significantly between countries (and companies) because of regulatory and other factors. For example, in the UK the Pharmaceutical Price Regulation Scheme has historically limited promotional and information costs to less than 50 per cent of the allowable research investment The World Pharmaceutical Market is presently ‘worth’ about $500 billion per annum World region Af rica + Asia + Australia Europe 4.2 Japan Latin America 10.7 USA + C anada 47.0 30.0 8.2 As already noted, innovative medicines typically have low marginal costs of production relative to their average costs. Disputes over what a ‘fair price’ is can stem from problems such as those relating to the allocation of research and development outlays The research based (bio) pharmaceutical industry has the highest ratio of research investment to income found in any sector. It is a major contributor to therapeutic innovation and bio-scientific development, and a vital partner for publicly funded researchers For the research based industry’s business model to remain viable, high investment risks must be counterbalanced by opportunities for above average profit. This in turn demands adequate intellectual property protection Parallel trading, patents and welfare In Europe different national authorities set varying medicine price/reimbursement levels. This has led to the ‘parallel trading’ of patented (and other) medicines within the EU ‘single market’ There has been debate in the US on the desirability of ‘low cost drug re-importation’ from Europe. But this could harm EU public interests. Such concerns highlight the complexity of issues relating to pharmaceutical intellectual property rights and the funding of private (and public) sector pharmaceutical research Byron Dorgan and Olympia Snowe In the UK a recent UK Social and Economic Research Council study found that although the UK is one of Europe’s largest parallel importers, the net impact on the British economy is probably negative There is research evidence from the US that reducing innovative medicine prices cuts research investment in a ratio of around 2:1 Patented medicine prices are above the EU average in the US, while those of generic drugs are relatively low. High absolute medicine spending in the US is also due to high use of newer medicines to treat conditions such as cancer Armatya Sen Conflicting pressures on medicine prices and spending Ernest Solvay and the structure of soda ash – Since the start of the 1950s health spending in Western Europe has increased from about 3 to around 10 per cent of GDP. Of this total, medicine costs typically account for a highly visible 10-20 per cent – Pharmaceutical research spending has risen consistently since the 1950s. But the number of marketed innovations has fallen and the age of high volume use ‘blockbusters’ appears to be drawing to a close – Particularly in EU Member States with weak pharmaceutical sectors, governments may subject to strong pressures to limit new medicine prices in ways which are not necessarily consistent with long term national/European public interests –There is in most markets a growing divergence between the prices of patented and non-patented medicines –All aspects of pharmaceutical research, testing, production and promotion are today heavily regulated. Nevertheless, it remains inherently costly to introduce new treatments and inform professionals as to their appropriate usage –Despite opportunities for further therapeutic gains, the use by agencies such as the UK’s NICE of incremental cost effectiveness analysis to assess the value/affordable price of new medicines may, in any given therapeutic area, make research on new treatments uneconomic in once very low cost generic medicines become available Questions for debate – To what extent (if any) would Belgian public interests be served by changes in pharmaceutical pricing that would further decrease the prices of off patent medicines relative to patented products? – To what extent (if any) would Belgian and European public interests be served by reforms that would reduce pharmaceutical parallel trading in Europe, or harmonise EU wide medicines pricing? – To what extent (if any) would Belgian and European public interests be served by reforms that could in future increase the duration of pharmaceutical patent protection in the EU or globally, balanced by appropriate changes in pricing and supply strategies? Conclusions To date Belgian approaches to pharmaceutical pricing and allied issues appear to have been consistent with public interests. But further national and/or EU wide reforms will in time be required Any future failure to take a constructive approach to fostering (bio)pharmaceutical science based industry and therapeutic innovation could well impose heavy costs on Europeans, and ultimately humanity as a whole In the modern context open and constructive public dialogue between the all the members of the complex network of stakeholders involved in pharmaceutical pricing and wider health policy formation and implementation is needed to protect against damaging errors. Belgium is well placed to lead 21st century Europe in this area [email protected]